-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(2): 21-29
doi:10.5923/j.ajb.20160602.01

Silymarin and Hydroethanolic Extracts of Silybum marianum Leaves and Fruits Attenuate Diethylnitrosamine/Phenobarbital-Induced Nephrotoxicity via Their Antioxidant and Anti-Inflammatory Actions
Osama M. Ahmed 1, Ayman M. Mahmoud 1, Sameh F. Abou Zid 2, Nour Y. Saber 1
1Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
2Pharmacognosy Department, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt
Correspondence to: Osama M. Ahmed , Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The current study was designed to evaluate the possible preventive effects of silymarin and hydroethanolic extracts of Silybum marianum leaves and fruits on diethylnitrosamine (DEN)/phenobarbital (PB)-induced nephrotoxicity in rats. The albino rats used in this study were allocated into 5 groups. Group I was kept as a normal control group, while the other 4 groups were administered a single intraperitoneal dose (200 mg/kg) of DEN, followed by daily PB-administration in drinking water from the 3rd week till the end of the experiment. Group II was DEN/PB-administered control group and groups III, IV and V were administered DEN and PB and were orally given silymarin, milk thistle leaves and milk thistle fruits extracts at a dose of 10, 50 and 50 mg/kg/day, respectively, for 14 weeks. DEN/PB-administrations induced kidney injury evidenced by histological alterations as well as significant increase of serum urea and creatinine concentrations. They also produced a significant increase in serum TNF-α level and a significant decrease in serum adiponectin level. On the other hand, the renal lipid peroxidation was elevated while renal glutathione content and activities of superoxide dismutase, catalase, glutathione peroxidase and glutathione-s-transferase were significantly declined as a result of DEN/PB administrations. Concomitant supplementation with silymarin and hydroethanolic extracts of Silybum marianum leaves and fruits markedly alleviated the altered biochemical and histopathological features. Thus, it can be concluded that silymarin and hydroethanolic extracts of milk thistle leaves and fruits successfully attenuate the DEN/PB-induced nephrotoxicity via their antioxidant and anti-inflammatory actions.
Keywords: Silymarin, Silybum marianum, Nephrotoxicity, Diethylnitrosamine, Phenobarbital, Antioxidant activity, Anti-Inflammatory effects, Rats
Cite this paper: Osama M. Ahmed , Ayman M. Mahmoud , Sameh F. Abou Zid , Nour Y. Saber , Silymarin and Hydroethanolic Extracts of Silybum marianum Leaves and Fruits Attenuate Diethylnitrosamine/Phenobarbital-Induced Nephrotoxicity via Their Antioxidant and Anti-Inflammatory Actions, American Journal of Biochemistry, Vol. 6 No. 2, 2016, pp. 21-29. doi: 10.5923/j.ajb.20160602.01.
1. Introduction
- The kidney plays a dominant role in homeostasis by excreting the metabolic waste products and excess necessary substances and it conserves necessary products depending on the needs of the body [1]. It is prime target of several drugs, toxic xenobiotic or chemicals due to high rate of blood flow (20%-25% of cardiac output) and presence of cellular transport systems that cause accumulation of these compounds within the nephron epithelial cells [2]. The drug or xenobiotic-induced nephrotoxicity or toxic effects may include altered inflammation, tubular cell toxicity, intraglomerular hemodynamics, crystal nephropathy, thrombotic microangiopathy and rhabdomyolysis [3, 4]. Nitrosamines, strong renalotoxic compounds, are formed endogenously from nitrate and nitrite under certain conditions such as the strong acidic pH of the stomach [5]. Diethylnitrosamine (DEN) is synthesized endogenously, is produced from metabolism of some drugs and is found in processed meats, tobacco smoke, soybean, cheese and wide variety of foods [6]. It was reported that DEN causes oxidative stress during the metabolism that leads to cytotoxicity, mutagenicity and carcinogenicity [7, 8]. DEN is biotransformed by mixed-function CYP450 dependent monooxidase systems and its metabolic activation is responsible for the onset of the toxic effects [9]. Oxidative stress has been reported to play a key role in the pathogenesis of drug-induced renal damage, and reactive oxygen species (ROS) have been implicated in the mechanisms that lead to tubular necrosis [10]. Hence, the use of antioxidants could offer protective effects against drug-induced renal damage. Silybum marianum, commonly known as milk thistle, is one of the oldest herbs used for centuries as an herbal medicine for the treatment of liver disease. It grows in rocky soils to a height of three to ten feet with an erect stem that bears large, alternating, prickly-edged leaves. Its flowering season is from June to August and each stem bears a single, large, purple flower ending in sharp spines. The fruit portion of the plant is glossy brown or grey with spots [11]. Silymarin, which was isolated from the milk thistle plant [12] has been used as a hepatoprotective substance for more than 2,000 years and is known to be non-toxic [13]. Over the last decade, numerous studies have shown that the main component of silymarin is silybin (SIL). SIL, exhibits anticancer and chemopreventive properties in various in vitro and in vivo models of various cancers, including lung [13], colorectal [14], breast [15], prostate [15], brain [16], ovarian [17], and kidney [18] cancers.In conduction with the previous publications, this study is designed to assess the preventive effects of silymarin and ethanolic extract of milk thistle on DEN/PB-induced kidney injury in male albino rats.
2. Materials and Methods
- Experimental animalsAdult male albino rats, each weighing about 100-120 g, were used in the present study. The animals were obtained from the National Institute of Ophthalmology, Giza, Egypt. They were kept under observation for two weeks before the onset of the experiment to exclude any intercurrent infections. The animals were housed in polypropyline well-aerated cages at normal atmospheric temperature (25±1°C) and normal 12-h light/dark cycle. Rats were provided with free access to water and were supplied daily with laboratory standard diet of known composition ad libitum. All animal procedures were undertaken with the approval of Institutional Animal Ethics Committee of Faculty of Science, Beni-Suef University (Egypt).ChemicalsAll chemicals were of analytical grade. N-nitrosodiethylamine (Synonym: Diethylnitrosamine, Product number: N0258, molecular formula: C4H10N2O, NDEA) is a yellow liquid dispensed in 1 ml ampoules. It was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Phenobarbital (PB) was purchased from Wako, Japan. Silymarin is present under the trademark (Legalon 140, Madaus AG, Koln, Germany). Pyrogallol, thiobarbituric acid (TBA), glutathione (GSH) and 5,5 dithiobis-(2-nitrobenzoic acid) (DTNB) were purchased from Sigma (USA).Plant materialsMilk thistle plant was collected from Beni-Suef governorate (Egypt) and was botanically authenticated by Dr. Mohamed Ahmed Fadl, lecturer of taxonomy, Botany Department, Faculty of Science, Beni-Suef University, Egypt.Induction of nephrotoxicity Nephrotoxicitywas induced by a single intraperitoneal injection of DEN at a dose of 200 mg/kg b.wt. Two-weeks later after DEN-administration, rats received 0.05% phenobarbital in drinking water for up to 12 successive weeks [19].Preparation of the milk thistle extractsMilk thistle leaves and fruits were separately washed in fresh water more than one time to remove the contamination. The washed leaves and fruits were air dried in shade area for 25 days, coarsely powdered and then were extracted with 70% aqueous ethanol for 72 hours at room temperature. The suspensions were stirred from time to time to allow the powder to fully mix with extraction solvent. The 70% aqueous ethanol extracts were filtered through Whatman filter paper and then evaporated under low pressure by using rotary evaporator to yield crude-dried extracts of milk thistle leaves and fruits.Dose preparation of silymarin and milk thistle leaves and fruits extractsSilymarin was dissolved in 1% carboxymethylcellulose (CMC) and was orally administered at a dose level of 10 mg/kg b.wt daily for 14 weeks. The dose of silymarin has been chosen according to the publication of El Sohafy et al. [20]. Milk thistle leaves and fruits hydroethanolic extracts were dissolved in CMC (1% w/v) and were orally administered at a dose level of 50 mg/kg b.wt. daily [21] for 14 weeks.Experimental Design The experiment was performed on fifty adult male albino rats randomly distributed into 5 groups, of ten animals each. Group I was kept as a normal control group, while the other 4 groups were administered a single intraperitoneal dose (200 mg/kg) of DEN, followed by daily PB-administration in drinking water from the 3rd week till the end of the experiment. Group II was DEN/PB-administered control group and groups III, IV and V were administered DEN and PB and were orally given silymarin, milk thistle leaves and fruits extracts, respectively, in a dose of 10, 50 and 50 mg/kg/day, respectively, for 14 weeks.Blood and kidney samplingBy the end of the experiment, animals were sacrificed under mild anesthesia. Blood samples were collected from jugular veins, left to coagulate and centrifuged at 3000 rpm for 15 min to separate the serum. Kidney samples were immediately excised and perfused with ice-cold saline. Frozen kidney samples were homogenized in chilled saline (10%wt/vol) and the homogenates were centrifuged at 3000 rpm for 10 min. The homogenate supernatants were collected and kept in deep freezer at –30°C until used for the determination of oxidative stress parameters and antioxidant defense markers.Biochemical assaysDetermination of serum creatinine and urea concentrationsSerum creatinine and urea concentrations were assayed using reagent kits purchased from Biosystems (Spain), following the methods of Fabiny and Ertingshausen [22] and Tabacco et al. [23], respectively. Determination of serumTNF-α and adiponectin levelsTNF-α and adiponectin levels were also determined by using ELISA kits purchased from R and D systems, USA according to the manufacturer’s instructions.Detection of kidney oxidative stress and antioxidant defense systemThe supernatants were used for estimation of lipid peroxidation (LPO) [24], reduced glutathione (GSH) content [25] and activities of kidney antioxidant enzymes including glutathione peroxidase (GPx) [26], superoxide dismutase (SOD) [27], catalase (CAT) [28] and glutathione-S- transferase (GST) [29]. All reagents were prepared in laboratory.Histopathological studyAfter sacrifice, decapitation and dissection, kidney from each rat was rapidly excised and then perfused in saline solution. Pieces from the kidney of rats of different groups were taken and fixed in 10% neutral buffered formalin for twenty four hours. Washing was done in tap water and serial dilutions of ethyl alcohol were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56°C in hot air oven for twenty four hours. Paraffin wax tissue blocks were prepared for sectioning at 4 microns thickness by slide microtome. The obtained tissue sections were collected on glass slides, deparaffinized, stained with hematoxylin and eosin (H&E) stains for routine examination done by the light electric microscope [30]. Statistical analysisResults were expressed as mean ± standard error (SE). The data were analyzed by one-way analysis of variance (ANOVA) using PC-STAT, University of Georgia, followed by LSD analysis to compare various groups with each other [31]. Values of P>0.05 were considered non-significantly different, while values of P<0.05 and P<0.01 were significantly and highly significantly different.
3. Results
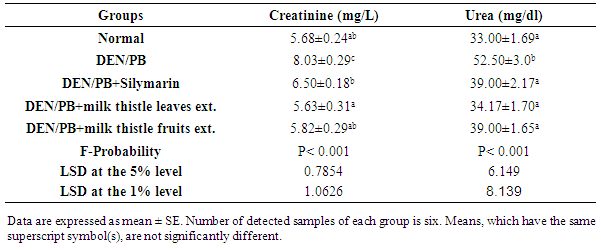
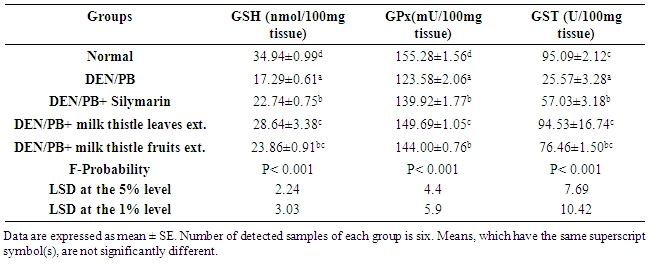
- Effect on the concentrations of creatinine and ureaDEN/PB-administration resulted in a marked impairment of kidney functions as demonstrated by the highly significant (P<0.01; LSD) increase of serum creatinine and urea concentrations. The treatment of DEN/PB-administered rats with silymarin and milk thistle leaves and fruits extracts induced a highly significant improvement (P<0.01; LSD) of serum creatinine and urea concentrations as compared to DEN/PB-administered rats. The milk thistle leaves extract seemed to be the most effective in improving the elevated creatinine and urea levels (Table 1).
|
|
|
|
4. Discussion
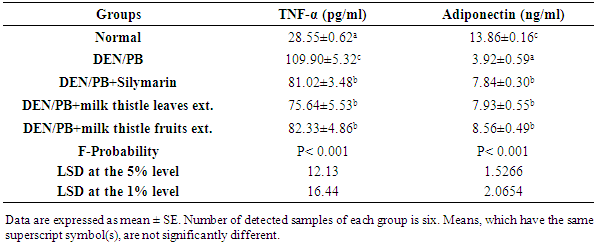
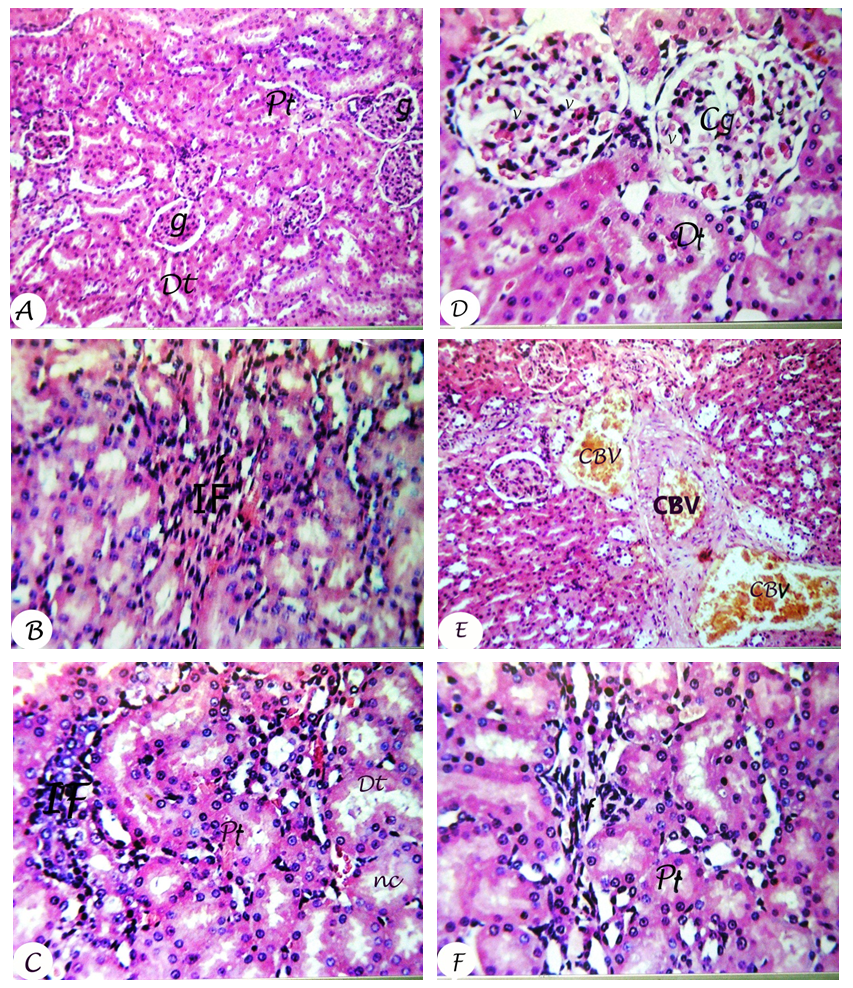
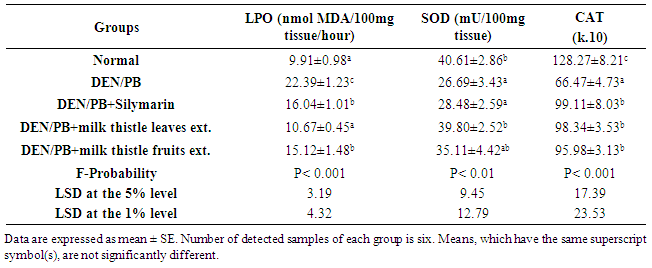
- Kidneys are pivotal in the elimination of numerous xenobiotics, including drugs and environmental chemicals, as well as endogenous metabolites. Kidneys have developed transport systems to prevent urinary loss of filtered nutrients, such as glucose, oligopeptides, and inorganic ions, as well as to facilitate the elimination of a variety of xenobiotics [32]. The present study showed that the administration of DEN/PB has induced renal damage and this was evident by the increased concentrations of the markers that have been used to assess renal function like creatinine and urea. It has been reported that serum creatinine concentration relates to glomerular function and its rise is an indicator of renal failure [33, 34]. These findings are in agreement with the studies of Rezaie et al. [35] and Pashmforoosh et al. [36] who demonstrated that the administration of DEN to rats increased the concentrations of creatinine and urea.On the other hand, the DEN/PB-administered groups treated with silymarin and milk thistle leaves and fruits extracts ameliorated the kidney function which is evidenced by the reduction of the elevated creatinine and urea concentrations. These improvements in the kidney function markers in serum were associated with marked amendment of kidney histological changes and integrity in the present study. TNF-α, a cytokine that plays a role in many inflammatory diseases, is produced by several pro-inflammatory cells (mainly macrophages, but also monocytes, dendritic cells, B-cells, CD+4 cells, neutrophils, mast cells and eosinophils) and structural cells (fibroblasts, epithelial cells and smooth muscle cells). TNF-α is a well-known inducer of the inflammatory response and a regulator of immunity. Its inflammatory properties are classically mediated by means of a wide variety of pro-inflammatory cytokines, including IL-1, IL-2, IL-6, IL-12 IFN-γ (interferon-γ) and TGF-β (transforming growth factor-β), generated mainly through NF-κB activation [37-39].TNF-α is a growth factor for most tumor cells [40]. It has been shown to mediate tumor initiation, promotion and metastasis [41]. The pro-inflammatory effect of TNF-α is due primarily to its ability to activate NF-κB. Almost all cell types, when exposed to TNF-α, activate NF-κB, leading to expression of inflammatory gene, cell adhesion molecules, inflammatory cytokines, chemokines, and inducible nitric oxide synthase [42].Adipokines are multifunctional secreted factors that are primarily considered to be derived from adipose tissue. Such tissue has been regarded simply as means of storing energy, whereas it is now considered to be a complex organ that is involved in the control of many processes including metabolic, immunological and inflammatory responses. Adiponectin is the most abundant and adipose tissue-specific adipokine. Mature adipocytes mainly produce adiponectin in white adipose tissue and expression and secretion levels increase during adipocyte differentiation [43]. On contrary to TNF-α, the administration of DEN/PB decreased the concentration of adiponectin in serum, while the treatment with silymarin and milk thistle leaves and fruits extracts increased its level. These results are in agreement with the previous publication which stated that pro-inflammatory adipokines such as TNF-α or IL-6 suppresses adiponectin, which has anti-inflammatory, antiatherogenic and antidiabetic properties [44]. These results of TNF-α and adiponectin levels go parallel with the present histological investigations which indicated inflammatory cell infiltration in kidney tissue of DEN/PB-administered rats and decrease or absence of inflammatory cells in DEN/PB-administered rats treated with silymarin and milk thistle leaves and fruits extracts. ROS are generated during the detoxification of xenobiotics and drugs, and cause oxidative stress. It is worth mentioning that oxidative stress has been shown to be linked to kidney toxicity and diseases. Oxidative stress is also associated with damage to a wide range of macromolecular species including lipids, proteins and nucleic acids. MDA is a major oxidation product of peroxidized polyunsaturated fatty acids, and increased MDA content is an important indicator of an increase in lipid peroxidation (LPO) [45, 46]. In the current study, kidney LPO was significantly elevated in response to the administration of DEN and PB. This may be due to an enhanced generation of superoxide radicals (O2.-) and hydrogen peroxide radicals that accelerated peroxidation of native membrane lipids. Peroxidation of cell and mitochondrial membrane led to a loss of cell integrity, increase in membrane permeability, and alternate Ca+2 homeostasis that contribute to cell death due to alteration in the inner membrane potential [45, 46]. In addition, some ROS interact with various tissue compounds leading to dysfunction and injury to the kidney and other organs [45, 46].The kidney injury is probably due to the deleterious effect of DEN itself and/or its metabolites which includes ethylcarbonium ions, NO and ROS such as superoxide radicals [47]. Ethylcarbonium ions bind to DNA forming adducts and generate superoxide radicals through lipid peroxidation of phospholipid membrane fatty acids [48].Protection against oxygen free radicals involves enzyme activities of CAT, SOD, GPx, glutathione reductase (GR), and non-enzymatic systems (vitamin E, vitamin C and GSH) of protection [49-52]. Balanced combination of the actions of antioxidant enzymes such as SOD, GPx and CAT is very important to achieve a better protection against oxidative stress damage [53]. Superoxide is the primary ROS produced in the course of oxygen metabolism which is highly reactive and cytotoxic. Superoxide is converted to a far less reactive product, hydrogen peroxide by a family of metalloenzymes known as SOD which constitute a front line of defense against ROS-mediated injury. Oxidative stress is the major cause for the development of chronic renal failure [54]. GPx is the most important antioxidant enzyme in humans which is highly expressed in the kidney, involved in scavenging and inactivating hydrogen and lipid peroxides, providing protection to the body against oxidative stress and also removes peroxides and peroxynitrite that can cause renal damage [55]. GPx with other selenoproteins containing selenocysteine play an important role in the GSH dependent defense against peroxynitrite-mediated oxidations by serving as a peroxynitrite reductase [56]. GSH is an important antioxidant tri-peptide in the cells, preventing damage to important cellular components caused by ROS such as free radical and peroxides [57]. GSH can detoxify many endogenous toxins, through the formation of GSH adducts to protect cells from the potential nephrotoxicity [58]. CAT and peroxidase are two common enzymes that catalyze the decomposition peroxides [59]. CAT and peroxidases as glutathione peroxidase are highly effective in inhibiting various ROS-mediated injuries and could protect the kidney from DEN-induced nephrotoxicity that occurred in the present investigation. In the present study, DEN/PB-mediated renal tissue injury increased kidney tissue LPO due to release of free radicals during nephrotoxic condition. The treatment with milk thistle leaves and fruits extracts reduced the kidney LPO and counteracted the formation of free radicals induced by DEN/PB-mediated nephrotoxicity; this effect displayed the protective role of these extracts in the prevention of renal damage in such condition. The standard drug silymarin also exhibited similar effect during nephrotoxicity.In present investigation, a striking decrease in these antioxidants (SOD, CAT, GSH, GPx and GST) in DEN/PB-exposed rats elicits strong evidence for the involvement of oxidative damage in DEN/PB-induced nephrotoxicity. The treatment with silymarin and the hydro-alcoholic extracts of the dried leaves and fruits of Silybum marianum produced a significant improvement of the decreased CAT, GSH, GPx and GST activities in the kidney of DEN/PB-intoxicated rats. In addition, renal injury induced by DEN was confirmed by the observed histological alterations that include focal fibrosis, necrosis and focal inflammatory cells infiltration in between the tubules, while the treatment of DEN/PB-administered rats with silymarin showed mild alterations represented by swelling vacuolization in the lining endothelium of the congested glomerular tufts. The rats treated with milk thistle leaves extract showed congestion in the blood vessels and the treatment with milk thistle fruits extract showed mild focal fibrosis between the tubules. Overall, the administration of DEN/PB induced glomerular dysfunction which was ameliorated by the three tested treatments. All these improvements revealed that the treatments used in the present study exert a great preventive effect against nephrotoxicity induced by DEN/PB-administration, and their preventive effects may be at least in part due to their antioxidant and anti-inflammatory properties. In conclusion, a possible preventive effect of silymarin and hydroethanolic extracts of milk thistle leaves and fruits on DEN/PB-induced nephrotoxicity may be explained on the basis of oxidant-antioxidant system management as well as regulation of the inflammatory status. Thus, these treatments may act as antioxidant and anti-inflammatory preventive agents. However, further clinical studies are required to assess the safety and the efficacy of these agents in human beings.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML