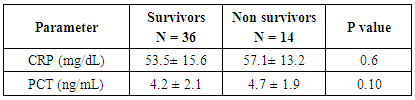-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(1): 6-15
doi:10.5923/j.ajb.20160601.02

Plasma Procalcitonin and Proadrenomedullin Concentrations as Predictive Markers for Early Onset Neonatal Sepsis
Ashraf T. Abd Elmouttaleb1, Hesham A. Aly2, Ebrahim M. Bayomy3, Mohamed R. Abdelhamed3, Nabil F. Esmael3
1Medical Biochemistry Department, Assisted Reproductive Unit, International Islamic Center for Population Studies and Research, Al-Azhar University, Egypt
2Pediatric Department, Faculty of Medicine, Al-Azhar University, Egypt
3Clinical Pathology Department, Faculty of Medicine, Al-Azhar University, Egypt
Correspondence to: Ashraf T. Abd Elmouttaleb, Medical Biochemistry Department, Assisted Reproductive Unit, International Islamic Center for Population Studies and Research, Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Adrenomedullin (ADM), a peptide with 52 amino acids, has immune- modulating, metabolic and vascular actions, it is mainly released from endothelial cells, epithelial cells of various origins and some other tissue-specific cells types. The mid-regional fragment of pro-adrenomedullin (MR-Pro-ADM), included between amino acids 45-92 is the most stable part of ADM, and it has been detected in the plasma of patients with septic shock as a consequence of the ADM active peptide degradation. Elevation of pro-ADM has been reported in systemic inflammatory response syndrome (SIRs), sepsis, and septic shock in adults. Aim of the work: Establishment the diagnostic utility (sensitivity, specificity, and predictive values) of plasma procalcitonin and proadrenomedullin in comparison to commonly used laboratory for sepsis (CRP, total leucocytic count), both individually and combination, for diagnosis of early onset neonatal sepsis. Subjects and methods: This study was included 80 newborns (50 newborn with neonatal sepsis and 30 control healthy newborns). The fifty septic cases all are full term and their gestational age ranged between 36-40 weeks, Plasma CRP concentrations were measured using an immunoturbidimetric method, Procalcitonin was measured by ELFA technique; enzyme linked fluorescent assay and Pro-adrenomedullin was measured using enzyme linked immuno sorbent assay (ELISA). Results: The mean plasma pro-adrenomedullin level in sepsis groups was (4.7±3.2) nmol/L which is significantly higher compared to control group (0.79±0.31)nmol/L with P<0.001. At a cut-off value 3.9 nmol/L the sensitivity of pro-ADM in detecting early onset neonatal sepsis was 91.6, its specificity was 87.4, its positive predictive value was 91.3, and its negative predictive value was 90.4, which represent highest sensitivity and specificity than the other studied markers. The plasma level of pro-ADM was significantly higher in non-survivor group with mean (8.8 ± 2.2) nmol/L compared to (2.6 ± 1.7) nmol/L in survived groups, while there were no significant differences of plasma CRP and Procalcitonin levels between survivors and non-survivors groups. Conclusions: Pro-adrenomedullinis sensitive, independent biomarker that can be used as a good tool for diagnosis of early onset neonatal sepsis, addition of procalcitonin slightly improve diagnostic accuracy by increasing sensitivity, specificity, predictive values and AUC. Pro-adrenomedullin might become a new promising prognostic marker for predicting the outcome of early onset neonatal sepsis as its plasma level was significantly higher in these patients with sepsis who did not survive than in survivor.
Keywords: Early neonatal sepsis, CRP, Procalcitonin, Proadrenomedullin
Cite this paper: Ashraf T. Abd Elmouttaleb, Hesham A. Aly, Ebrahim M. Bayomy, Mohamed R. Abdelhamed, Nabil F. Esmael, Plasma Procalcitonin and Proadrenomedullin Concentrations as Predictive Markers for Early Onset Neonatal Sepsis, American Journal of Biochemistry, Vol. 6 No. 1, 2016, pp. 6-15. doi: 10.5923/j.ajb.20160601.02.
1. Introduction
- Neonatal sepsis in defined as an invasive bacterial infection which occurs in the first weeks of life. Bacterial invasion caused from a local infectious source into blood stream leads to signs of systemic illness in remote organs [1]. Two patterns of disease of early onset (<7days birth), eighty five percent of newborns with early onset infection present within 24hours: on the other hand late onset sepsis occurs at 7-28 days of life and it is more insidious in presentation with lower mortality [2]. World health organization (WHO) estimates that out of the four millionneonatal deaths all over the world every year, over 35% is due to infection in the neonatal period. Newborns of whole world especially those of the third world countries are the most vulnerable group for this illness. It is crucial to protect the newborns from infection as far as possible. In a poor country like ours, it is the responsibility of pediatricians (beside neonatologists) to address this serious neonatal health problems: rates of sepsis exceeding 50% in a neonatal intensive care unit (NICU) in Cairo, Egypt, were not controlled by routine antimicrobial therapy [3]. Although great progress has been made in the treatment of sepsis, the mortality of patients with severe sepsis is still as high as 30%-70%. Moreover high expenditure of sepsis management has resulted in a heavy financial burden to the government and patients. Therefore how to estimate the severity of sepsis early and apply targeted therapies timely is very important in the treatment of sepsis [4]. Blood culture is a gold standard for diagnosis of infection; ideally antibiotic therapy should be directed against the causative organism. However, it takes too long time, the result is available within 24-72 hours and the rate of false negative results is relatively high, bacteremia is identified in only about 30% of patients with sepsis. Beside it is impractically to obtain blood sample for serial blood cultures from infants. So, new laboratory methods for early diagnosis of the disease, evaluation of prognosis and treatment efficiency are needed [5]. Many biomarkers can be used in sepsis, but none has sufficient specificity to be routinely employed in clinical practice. No single biomarker of blood stream infection may be ideal, but many are helpful in terms identifying bacterial infections in critically ill patients who need close monitoring [6]. C-reactive protein (CRP) is a globulin protein produced in response to infection and/or inflammation and it is widely used in clinical tests to diagnose and mange patients with sepsis. This biomarker is an acute phase reactant whose synthesis in the liver is up regulated by IL-6; because the level of CRP rise significantly higher during acute inflammation, this biomarker has been used for decades to indicate the presence of significant inflammatory or infectious disease, especially in paediatrics [7]. CRP is a good marker for diagnosis of neonatal sepsis. Elevated CRP levels are seen in infection, autoimmune disease, surgery, meconium aspiration and recent vaccination. So, CRP does not reliably differentiate between systemic inflammatory response and sepsis. Also CRP cannot be used to differentiate between bacterial and other infection [8]. C-reactive protein (CRP), white blood cell count, absolute neutrophil count, are the most widely tests in the diagnosis of sepsis; all provide useful information, but none of them has been demonstrated to be reliable in detecting all septic infants. This has led to the search for new diagnostic marker [9]. Procalcitonin (PCT) is a propeptide (precursor) of calcitonin with 116 amino acid, it is formed of glycoprotein secreted by the C-cells of thyroid gland in normal situation, but its level may increase in severe infections like septicemia, meningitis, pneumonia and urinary tract infection [10]. Although the exact sites of production of PCT in sepsis have not been recognized, monocytes are believed to be potential sources. Serum concentration of PCT begins to rise four hours after exposure to bacterial endotoxin, peak at 6-8 hrs and remains raised for at least 24hrs. The half-life of PCT is longer than CRP and is estimated to be about 25-30hrs. Bacterial lipopolysaccharide (LPS) has been shown to be a potent inducer of PCT release into systemic circulation [11]. Procalcitonin has been claimed to be superior to other acute phase proteins including CRP with higher sensitivity and specificity than CRP, PCT levels rise earlier and return to normal levels more rapidly than CRP [12]. Infants with viral infection, non-infected inflammatory stress like birth trauma, aspiration syndrome and hypoxemia have normal or slightly elevated concentrations [12]. Many recent studies also considered PCT to be a superior marker of neonatal sepsis compared to CRP with the added advantage of differentiating bacterial and viral infection [13].Adrenomedullin (ADM), a peptide with 52 amino acids, has immune modulating, metabolic and vascular actions, it is mainly released from endothelial cells, epithelial cells of various origins and some other tissue-specific cell types [14]. It is a potent vasodilator, and its wide spread production in tissues helps to maintain blood supply to individual organs, other ADM properties include reduction in endothelial permeability, natriuretic effects, down--regulation of pro-inflammatory cytokines, and has bactericidal activity which further enhanced by its regulation and mediation of complement activity [15]. Unfortunately, the measurement of ADM is technically challenging and reliable measurement is almost impossible because it is rapidly cleared from circulations (short half- life of 22 minute). In addition, circulating ADM is masked by binding protein (complement factor H), making it in accessible for immunometric analysis [16]. Recently, the midregional fragment of pro-adrenomedullin (MR-Pro-ADM), included between amino acids 45-92 is the most stable part of ADM, and it has been detected in the plasma of patients with septic shock as a consequence of the ADM active peptide degradation [17]. ADM is involved in a variety of processes, including embryogenesis, normal and malignant growth, inflammation, and immunity. Plasma concentrations of ADM are increased in various disease processes, such as arterial hypertension, heart failure, acute coronary syndrome, renal failure, and septic shock [18]. Elevation of pro-ADM has been reported in systemic inflammatory response syndrome (SIRs), sepsis, and septic shock in adults [19].
2. Subjects and Methods
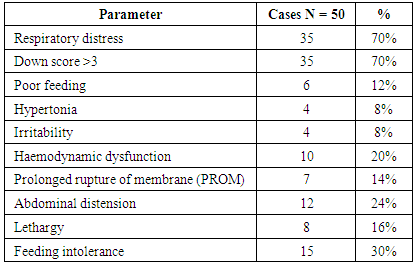
- This study included 80 newborns (50 newborn with neonatal sepsis and 30 control healthy newborns). The fifty septic cases all are full term and their gestational age ranged between 36-40 weeks. They were admitted to Bab El-Sharyia Hospital (neonatal intensive care Unit) during a period from March to September 2015. Sepsis was clinically diagnosed by presence for at least 3 of non-specific sign of sickness 1- fever or hypothermia 2- tachycardia or bradycardia 3- hypotension 4- tachypnea 5- chest retractions 6- grunt 7- lethargy 8- poor feeding 9- hypotonia 10- irritability 11- central cyanosis 12- shock 13- abdominal distension 14- down score >3. Gestational age was from last menstrual cycle, early fetal ultrasound and new Ballard score.Exclusion criteria: Infants with neonatal asphyxia, aspiration syndrome, metabolic disease, congenital malformations, infants of diabetic mothers, administration of antibiotic therapy prior to admission and negative blood cultures.Aim of this study (1) to determine the relation between plasma pro-calcitonin, and pro-adrenomedullin levels in early onset neonatal sepsis. (2) to establish the diagnostic utility (sensitivity, specificity, and predictive values) of plasma procalcitonin and proadrenomedullin in comparison to commonly used laboratory for sepsis (CRP, total leucocytic count), both individually and in combination, for diagnosis of early onset neonatal sepsis. (3) to test whether plasma procalcitonin and pro-adrenomedullin levels have any prognostic values for diagnosis of early onset neonatal sepsis.Subjects were enrolled in this study after a written informed consent from a parent.Control criteria: Thirty apparently healthy newborns served as control with gestational age and birth weight were matched with the studied septic infants. Two groups were prospectively defined. Group I: 50 full-term newborns with criteria of neonatal sepsis, their ages ranged from 1-6 days. Their gestational ages ranged from 37-40 weeks with positive blood culture results. Group II: The control group consisted of 30 healthy full term newborns. Their ages ranged from 1-6 days.Sampling: Venous blood samples were taken during first 24 hours after admission before starting antibiotic therapy. Each blood sample was distributed into plain, EDTA and heparintubes for measurement of standardbiochemical and hematological parameters, Blood gases, Blood culture, CRP, PCT and pro-adrenomedullin levels. EDTA tubes were centrifugated and the separated plasma were stored at 80° until the time of analysis. CRP, CBC, serum chemistry and blood gases were measured immediately.- Complete blood count (CBC) was measured by an automatic cell counter (Abbott cell-dyna-1700 hematology analyzer, Abbott, laboratories, North Chicago, Illinois).- Blood chemistry was measured by fully automated Hitachi (912) analyzer with commercial kit of Rochecompany.- Blood gases were measured by (COBAS 221, Roche, Gmbh, Germany).- Plasma CRP concentrations were measured using an immunoturbidimetric method (Haman Diagnostic, Wiesbaden, Germany) and quantified with a spectrophotometer. The detection limits of the assays for CRP were 0.1mg/l. References values for healthy neonates, using quantitative techniques are less than 1.6mg/dl during the first 2 days of life and less than 1mg/dl for remainder of neonatal period.- PCT was measured by ELFA technique; enzyme linked fluorescent assay using B.R.A.H.S. PCT (BioMerieux SA, France) kit by VIDAS equipment. The assay principle combines, a one-step immunoassay sandwich method with a final fluorescent detection. Reagents for assay are ready to use and pre-dispended in the sealed reagent strips. All the assay steps are performed automatically by the VIDAS instrument. The minimal detection limit is 0.05ng/ml.- Measurement of plasma pro-adrenomedullin: pro-ADM was measured using enzyme linked immuno sorbent assay kit. Briefly, the assay employs two polyclonal antibodies which are designed to detect midregional-pro-adrenomedullin (MR-pro-ADN) peptides on the basis of the principle of competitive enzyme immune assay, using (DRG International Inc., USA) kit. The minimal detection range is 0.33nmol/L.Microbiology examination:One or two ml blood was added to blood culture media and incubated at 37°C for 5-7 days. Bottles with positive results were sub cultured on blood agar and EMB media. Isolated microorganisms were identified by standard bacteriological methods. Blood cultures were positive for all patients group I.The identified bacteria included:Staphylococcus aureus (12 cases), Streptococcus pneumonia (9 cases), Streptococcus pyogenes (6 cases), Pseudomonas aeruginosa (5 cases), Staphylococcus epidermidis (4 cases), Acinebacter (5 cases), Klebsiella pneumonia (4 cases), Enterobacter spp (3 cases), and Escherichia coli (2 cases).Statistical analysis:The SPSSsoftware was used to data management and analysis. The quantitative data were presented as mean ± standard deviation. For comparison of two group's variable Man Whitney test was used. To study the relationship between two variables, spearman's correlation coefficients were used. Pvalue was carried to be significant if it was <0.05. The reliability of plasma CRP, PCT and pro-adrenomedullin concentrations for diagnosis of early onset neonatal sepsis were calculated by receiver operating characteristic curves (ROC) and the area under the curve (AUC) calculated. The (ROC) curve was used to predict the optimal cut-off values. The sensitivity, specificity and predictive values were calculated for CRP, PCT and pro-adrenomedullin using the cut-off values derived from ROC analysis.
3. Results
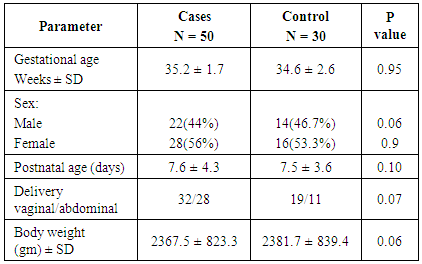
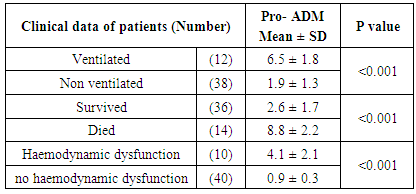
- In table (1): There were no statistical significant differences between both groups in demographic parameters.
|
|
|
|
|
|
|
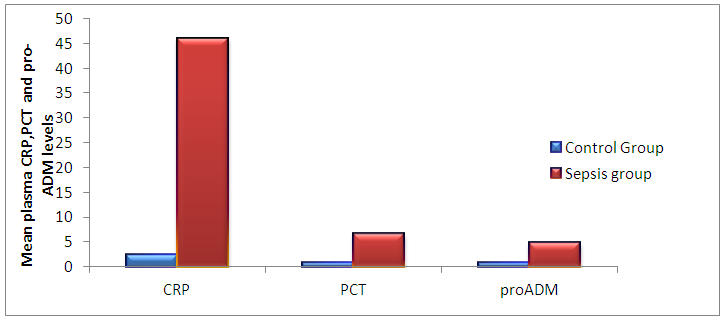 | Figure (1). The mean plasma CRP, Procalcitonin and Pro-adrenomedullin levels in control and sepsis groups |
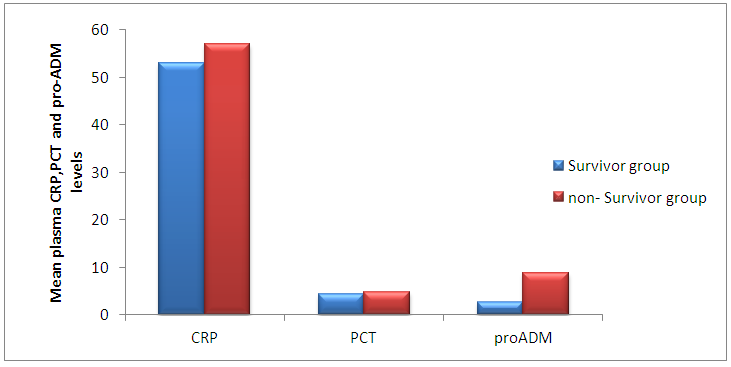 | Figure (2). The mean plasma CRP, Procalcitonin and Pro-adrenomedullin levels in survivors and non-survivors groups |
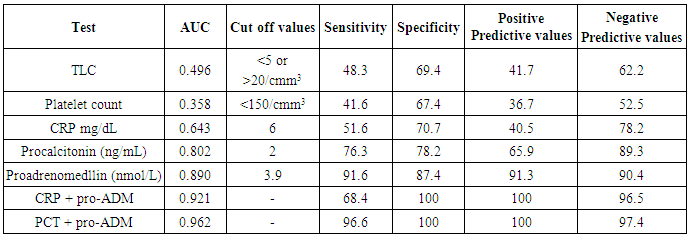
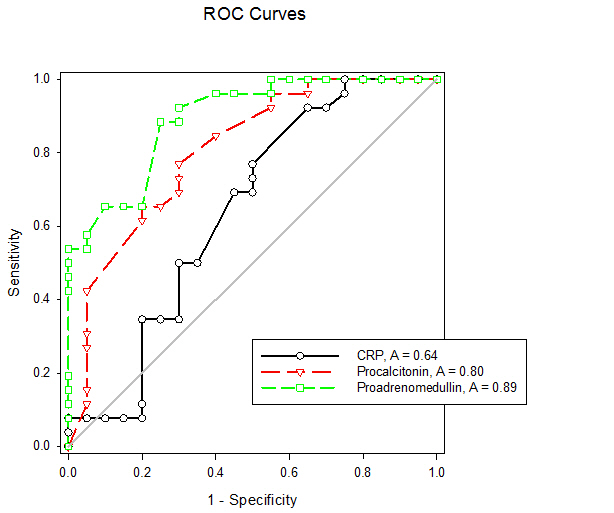 | Figure (3). Receiver operating characteristic curves of CRP, Procalcitonin and Pro-adrenomedullin for diagnosis of early onset neonatal sepsis |
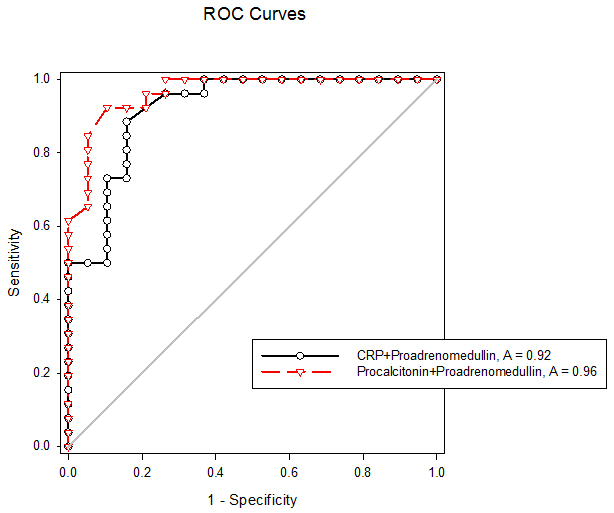 | Figure (4). Receiver operating characteristic curves for combination of CRP + Pro-adrenomedullin and Procalcitonin + Pro-adrenomedullin for diagnosis of early onset neonatal sepsis |
4. Discussion
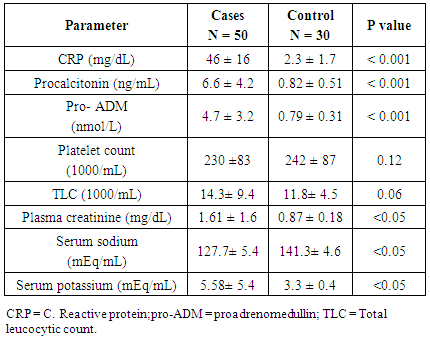
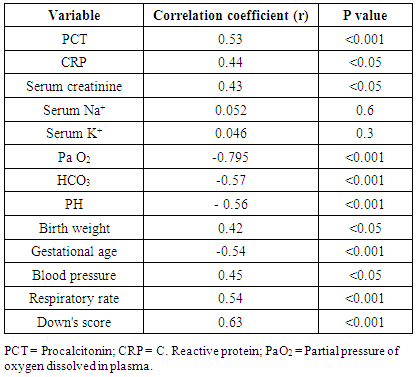
- Early onset neonatal sepsis (EONS) is one of the leading causes of neonatal morbidity and mortality-particularly in preterm infants, because the signs and symptoms of (EONS) are non-specific, a variety of non-infectious conditions can occur together with neonatal infection or can make the diagnosis of infection more difficult [20]. Early and accurate diagnosis and risk assessment are pivotal to optimal care of critically ill patients [21]. Sepsis is a complex syndrome including coagulation disorder, inflammatory response, cellular disorder and metabolic alteration [22]. An ideal sepsis marker should permit early diagnosis, should inform about the course of disease, and should help one to differentiate bacterial from non-infectious and viral causes of systemic inflammation, not only in sepsis trials but also in routine clinical application [23]. C-reactive protein (CRP), white blood cell count, absolute neutrophil count, and immature/total neutrophil ratio are the most used tests in the diagnosis of sepsis, all provide useful information but none of them has been demonstrated to be reliable in detecting all septic infants. This leads to a continuing search for new diagnostic markers [24].In the present study at a cut-off value 6mg/dL the sensitivity of CRP in detecting sepsis was 51.6, its specificity was 70.7, its positive predictive value was 40.5% and its negative predictive value was 78.2. These results demonstrated that plasma CRP has a moderate diagnostic efficiency in the detection of early neonatal sepsis.These results concede with the results of other studies showing limited accuracy of CRP in detecting neonatal sepsis [25, 26, 27].Biomarkers are becoming the way to improve diagnostic accuracy of early neonatal sepsis. PCT is a debatable biomarker compared to CRP for diagnosis of neonatal sepsis. Some studies have shown that PCT is more reliable than CRP for the diagnosis of early-onset neonatal sepsis, but other studies have not found any advantage of PCT over CRP. In our study at a cult-off value 2ng/mL the sensitivity of PCT in detecting neonatal sepsis was 76.3, its specificity was78.2, its positive predictive value was 65.9, and its negative predictive value was 89.3.From these results, PCT sensitivity and specificity were higher than those of CRP, PCT level was superior to CRP in terms of an early diagnosis of neonatal sepsis, but it was not sufficiently reliable to be used as a sole marker of neonatal sepsis. These findings confirmed previous results of other investigators who demonstrated that PCT was more sensitive than CRP in detecting neonatal sepsis. Altunhan et al. [28] concluded that, serial measurement of PCT at birth and at 24hrs of life may be helpful in differentiating between early onset neonatal sepsis and these non-infectious conditions, he considered that during the first 24hrs of life PCT is a more sensitive marker of infection than CRP. Carol et al. [29] showed that PCT is more sensitive than CRP in the diagnosis of septicemia, meningitis and urinary tract infection, Koksal et al. [30] reported that plasma PCT level was superior to plasma CRP level in terms of early diagnosis of neonatal sepsis, in detecting the severity of illness and in evaluation of the response to antibiotic therapy, arising PCT level is used as an indicator that an infectious process is not under control and that better source control is required. Moreover in the study of Lopez et al. [31] plasma PCT concentration showed a moderate diagnostic value for the detection of sepsis of vertical transmission, with better results after 12hrs of birth. In accordance with our study, the study of Sucilathangan et al. [27] who concluded that the plasma level of PCT is a more reliable marker than CRP level or the WBC count in the early diagnosis of neonatal sepsis.Also our results agree with the study of Zahedpasha et al. [32] who showed that PCT levels were remarkably high in neonate with proven sepsis and the levels dropped dramatically after treatment with antibiotics.In addition, similar results have been obtained by many authors that PCT is more specific and is detected faster than CRP [10, 33-37].Our results disagree with the results of other studies demonstrating that PCT sensitivity and specificity were lower than CRP sensitivity and specificity. In the study of Park et al. [38] CRP is founded to be a highly accurate marker, whereas PCT moderately accurate marker for diagnosis of neonatal sepsis.Blommendahl et al. [39] reported that PCT test appeared to be useful for the diagnosis of neonatal sepsis, but did not offer any significant advantages over traditional test like CRP for the diagnose of sepsis.Enguix et al. [40] demonstrated that both markers were seen to be similar in the diagnosis of neonatal sepsis, Janota et al. [41] indicated that the sensitivity and specificity of PCT were less than that of CRP, and PCT rise caused by prenatal events other than infection such as perinatal asphyxia and other conditions such as intracranial hemorrhage, pneumothorax or after resuscitation, these conditions had negatively affected the specificity of PCT.Monneret et al. [42] concluded that PCT elevation is common in respiratory distress syndrome and condition related to temporal hypoxia during labor, so high PCT level does not mean the bacterial infection all the time.Ballot et al. [43] suggested that the PCT test alone was not sufficient to confirm neonatal sepsis, because of its slightly lower sensitivity, specificity, and positive predictability, with additional measurement of CRP may increase the specificity.In the current study at a cut-off value 3.9nmol/L the sensitivity of pro-ADM in detecting early neonatal sepsis was 91.6, its specificity was 87.4, its positive predictive value was 91.3, and its negative predictive value was 90.4, which represent highest sensitivity and specificity of the studied markers.In this study the mean plasma proadrenomedullin level in sepsis groups was (4.7±3.2) nmol/L which is significantly higher compared to control group (0.79±0.31) nmol/L with P<0.001.Christ-Crain et al. [44] had explained increased pro-ADM in sepsis by different mechanisms, first, as a member of calcitonin receptor- like gene family; ADM is widely expressed and extensively synthesized during sepsis. Bacterial endotoxins and pro-inflammatory cytokines up regulate ADM gene expression in many tissues. Second, decrease clearance by the kidney may be responsible for the increased level in sepsis. Third, an alternative site of pro-ADM clearance may be the lung, and thus in infection related lung injury, impaired removal of pro-ADM from pulmonary circulation results in increased pro-ADM plasma levels. Our results are in agreement with the results of Mehmet Yekata et al. [45] in the study done on patients with clinical and proven sepsis compared to healthy control group, and revealed significantly higher levels of pro-ADM as compared with those of healthy controls in patients with either proven or clinical sepsis. Furthermore, patients with proven sepsis also had significantly higher levels of pro-ADM as compared with those with clinical sepsis.Also our results agree with the results of Christ-Crain et al. [44] who measured pro-ADM in patient with systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis and in patients with septic shock, it was founded that pro-ADM is significantly higher in all sepsis groups compared to those healthy controls and critically ill patients without infection. In addition, circulating mid-regional- pro-ADM levels on admission exhibited a gradual increase with increasing severity of sepsis. The study of Adamty et al. [46] reported that plasma mid-regional-pro-ADM concentrations are elevated in neonates, especially those born very preterm, and their increased concentrations are associated with immaturity and inflammation, the elevated MR-proADM concentrations may be causally linked to reduced systemic vascular resistance observed in these two conditions. In addition, the blood pressure increases during first days with drops in ADM unless there is diastolic run off through a patentducts arteriousus. In the study done by Miguel et al. [9] cord blood pro-ADM levels of newborns born after high-risk pregnancies deliveries were compared with healthy controls, a statistically significant difference was not observed. In the current study the levels of CRP and procalcitonin were not significantly different between survivors and non- survivors, on the other hand the plasma level of pro-ADM was significantly higher in non-survivor group with mean (8.8± 2.2) nmol/L compared to (2.6± 1.7) nmol/L in survived groups, this is consistent with the study of Christ-Crain et al. [44] which reported that the mean plasma Mid-regional-pro-ADM levels were significantly higher in those patients with sepsis who did not survive than in survivors, and concluded that the prognostic accuracy tended to be superior to those other biomarkers such as CRP and PCT, similar results were obtained by Hagag et al. [47] who found that pro-ADM and anti-thrombin levels were significantly lower in survived in contrast to non-survivor cases, on the other hand CRP was not significantly different between survivors and non-survivors. Wang and Kong 2010 [48] clarified that, on the first day of admission to the ICU, the levels of preatrial natriuretic peptide (pro-ANP) and pro-ADM in patients with sepsis, sever sepsis, or septic shock were significantly increased in non-survivors as compared with survivors, while other markers of infection and inflammation such as CRP, IL-6 and PCT were not significantly different suggesting that pro-ANP and pro-ADM are better in predicting the severity of sepsis patients.Recently circulating mid-regional pro-adrenomedullin (MR-pro-ADM) and carboxy terminal pro-endothelin-1 (CR-ProET-1) are peptides that are co-synthesized with ADM and Endothelin-1, respectively, and have the advantage of a longer half-life, lack of bioactivity and lack of protein binding, which makes them more suitable for daily practice [49].High levels of pro-adrenomedullin (MR-pro-ADM), carboxy terminal pro-endothelin-1 (CR-ProET-1), and PCT in critically ill children were associated with increased prediction of mortality risk score as well as increased risk of number of organ failure [50].In the current study the combination of CRP and pro-ADM has increased the specificity and predictive values but decreased the sensitivity of pro-ADM in diagnosis of early onset neonatal sepsis. This is explained by the increase in the serum concentration of CRP is rather slow during the first 24-48 hour of infection and this may negatively affect the sensitivity of pro-ADM, thus, CRP can be considered as specific but a late marker of neonatal sepsis.This result is supported by another study that demonstrated the increased CRP levels after the beginning of inflammation is slower than that in PCT levels. This difference is attributed to CRP starting to increase 4-6 hours later than PCT after beginning of inflammation, and reaching its peak about 36 hours later [51].In our study the combination of procalcitonin and pro-adrenomedullin has increased the sensitivity, specificity and predictive values with 100% specificity and100% positive predictive value. So, this combination improves diagnostic efficiency of pro-adrenomedullin and PCT for the diagnosis of early onset neonatal sepsis.
5. Conclusions
- 1. PCT concentration showed a moderate diagnostic value for detection of early onset neonatal sepsis.2. Pro-adrenomedullin alone is sensitive, independent biomarker that can be used as a good tool for diagnosis of early onset neonatal sepsis, addition of procalcitonin slightly improve diagnostic accuracy by increasing sensitivity, specificity, predictive values and AUC, on the other hand, addition of CRP lower the sensitivity of pro-adrenomedullin for diagnosis of sepsis thus limiting this combination for diagnosis of early onset sepsis.3. Pro-adrenomedullin might become anew promising prognostic marker for predicting the outcome of early onset neonatal sepsis as its plasma level was significantly higher in these patients with sepsis who did not survive than in survivors. This finding was not obtained by either CRP or PCT as both were not significantly different between survivors and non-survivors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML