-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2015; 5(5): 113-129
doi:10.5923/j.ajb.20150505.04

Evaluation of Gonado-Protective Potentials of Kigelia Africana (Lam.) Benth in Arsenic - Induced Gonadotoxicity in Male Wistar Rats
Bakare O. S.1, Areola J. O.1, Ayannuga O. A.2, Babalola O. O.1
1Department of Biochemistry, Faculty of Science, Obafemi Awolowo University, Ile-Ife, Nigeria
2Department of Anatomy, College of Health Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence to: Babalola O. O., Department of Biochemistry, Faculty of Science, Obafemi Awolowo University, Ile-Ife, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study investigated the phytochemical constituents of the ethyl acetate fraction of Kigeliaafricana,and studied its effects in ameliorating arsenic-induced gonad toxicity in male wistar rats. This was with a view to investigate its gonadoprotective potentials. The stem bark of Kigeliaafricana, were air-dried at room temperature for 30 days and were mechanically ground into fine powder by using an electrical mill. Seven hundred grams of powdered plant material was suspended in six litres of 80% (v/v) of methanol for 72 h followed by evaporation to dryness under reduced pressure and partitioned with hexane (HF), ethylacetate (EAF) and butanol (BF) to obtain the solvent fractions. The total phenolics and flavonoid contents of the methanolic extract and the fractions (HF, EAF, BF and AqF) were determined using standard methods while the antioxidant capacities of the extract/fractions were evaluated using: Ferric reducing power (FRAP) assay, total antioxidant assay, 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH) radical scavenging assay and metal chelating assay. The invitro anti-inflammatory potential of the extract/fractions was evaluated by determining its ability to protect red blood cells exposed to both hypotonic and heat-induced lyses. Gonadotocixity was induced by treating the animals with 3 mg/kg body weight of arsenic trioxide while the gonadoprotective potential of Kigeliaafricana was carried out by administering the extract and ethyl acetate fraction of plant material for 30 days at doses of 250 and 300 mg/kg and 300 mg ascorbic acid as positive control. Blood samples were collected by ocular puncture and the testicular organs of the rats were collected after sacrifice for hormonal study. The homogenates were assayed for alkaline phosphatase (ALP), acid phosphatase (ACP), lactate dehydrogenase (LDH) and glucose-6-phosphate dehydrogenase (G-6-PDH) activities. Superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and glutathione peroxidase (GPx) were measured in the testes using standard procedures. Sperm morphology and the histology of the testes were also studied. There was significant (p<0.05) decrease in the level of serum testosterone (1.0863 ± 0.01575 nmol/L), LH (70.22 ± 5.46 mIU/mL) and FSH (36.58 ± 1.03 mIU/mL) in arsenic treated rats compared to control (1.10 ± 0.0032 nmol/L), (150.28 ± 35.04 mIU/mL) and (40.31 ± 8.25 mIU/mL) respectively. The histology of the testes showed varying degree of cellular degeneration and necrosis rats treated with arsenic. However, the toxic damages were not restored with treatment with K.africana extract/fraction. The result showed that there was reduction in the sperm count of all the treated groups. The study concluded that arsenic trioxides caused damage to the gonads, while the methanolic extract and ethyl acetate fraction of stem bark of Kigeliaafricana did not show any appreciable degree of protection to the gonads at 250 and 500 mg/kg bwt used. Therefore Kigeliaafricanacould not be used in the treatment of sexual dysfunction.
Keywords: Kigeliaafricana, Testes, Sperm, Phytochemical, Antioxidant
Cite this paper: Bakare O. S., Areola J. O., Ayannuga O. A., Babalola O. O., Evaluation of Gonado-Protective Potentials of Kigelia Africana (Lam.) Benth in Arsenic - Induced Gonadotoxicity in Male Wistar Rats, American Journal of Biochemistry, Vol. 5 No. 5, 2015, pp. 113-129. doi: 10.5923/j.ajb.20150505.04.
1. Introduction
- The use of metals has been critical to the progress and success of human civilization [1]. It would be difficult to imagine an advanced society without extensive utilization of metallic compounds. Metals are unique among environmental toxicants in that they are all naturally existing and in many cases, are ubiquitous within the human environment. In addition, all life has evolved in the presence of metals and organisms have been forced to deal with these potentially toxic, yet ever present elements [2]. The rapid industrialization, uncontrolled urbanization, and the effects of environmental chemicals including heavy metals on male reproductive system have become major health concern globally [3] [4]. It is well known that good quality semen is essential for appropriate reproduction. This quality appears to have been negatively directly affected in recent years. This significant drop and consequently increase in male infertility rates [5] [6] [2] can be attributed to chemical and heavy metal exposure [7]. In the past few decades, tens of thousands of metals and chemicals have been released into the environment [8]. Over exposure to these metals has been reported to cause several anomalies associated with the male reproductive health [2]. Metals are everywhere, in food, dietary supplements, water, air, alcoholic drinks, and tobacco. Cigarette smoke contains about 30 metals, of which cadmium, arsenic, and lead are in the highest concentrations, and cadmium body burden in smokers is about double that of non-smokers [9]. Alcoholic beverages including wine can be contaminated with metals in concentrations exceeding the allowable limits causing toxic effects, particularly in heavy drinkers [10]. Human are inevitably exposed to metals due to its ubiquity in nature, wide use in industry and long-term persistence in the environment. Response to metal exposure depends on the age, sex, health status, and dietary habits, use of medications and/or supplements, physical activity, and concomitant exposure to other metals and/or chemicals [11] [12]. Among the heavy metals, arsenic exhibits a complex metabolism and is possibly the most abundant and a potential carcinogen [9]. Arsenic is a metalloid that is widely distributed in the environment in inorganic trivalent (inorganic arsenite; AsIII) or pentavalent (inorganic arsenate; AsV) forms, [13].It is a naturally occurring element which is found throughout the environment [13]. Arsenic is present in the nature in stable form as Ar5+ and As3+ species.In the process of arsenic metabolism, inorganic arsenic is methylated to monomethyl arsenic acid (MMA) and finally to dimethyl arsenic acid (DMA) followed by a renal excretion. In this process of biomethylation, constant depletion of methyl causes DNA hypomethylation, and thus generates mutation followed by carcinogenesis [9]. Arsenic affects the mitochondrial enzymes, impair the cellular respiration and causes cellular toxicity. It can also substitute phosphate intermediate, which could theoretically slow down the rate ofmetabolism and interrupt the production of energy [14]. Male infertility is reflected by low sperm count, low sperm motility and bad quality of sperms [14]. Sodium arsenite has been found to have an inhibitory effect on the activity of testicular steroidogenic enzyme ∆5-3β-hydroxysteroid dehydrogenase (∆5-3β-HSD) and 17β-hydroxysteroid dehydrogenase (17β-HSD) and to reduce the weight of testes and accessory and accessory sex glands in rats [14]. High Arsenic level may suppress the sensitivity of gonadotroph cells to GnRH as well as gonadotropin secretion by elevating plasma levels of glucocorticoids. These ultimately lead to the development of gonadal toxicity [14].
2. Materials and Methods
- Collection and Identification of Plant MaterialsFresh stem bark samples of K. africana were collected from Iperindo, in Atakumosa East Local Government Area of Osun State, Nigeria. The plantwas identified and authenticated by Mr. G. A. Ibhasebor at IFE Herbarium Department of Botany, Obafemi Awolowo University, Nigeria. The Herbarium identification number was IFE 17219.Extraction and Preparation of Methanolic Extract of K. africanaThe stem bark of K. africana were air-dried at room temperature for thirty (30) days and were mechanically ground into fine powder by using an electrical mill. The powderwas kept in air-tight container until use. Six hundred (600 g) of the dried powders was soaked in 4 litres of 80% (v/v) of methanol for 72 h with occasional agitation using sterile rod. The mixture was filtered using muslin cloth followed by Whatman filter paper (No. 1). The resultant filtrate was evaporated to dryness under reduced pressure using a rotary evaporator at 40°C (Buchi) to give a dark brown extract. Partitioning of Crude Methanolic Extracts of K. africana Stem BarkThe crude methanolic extracts of K. africana stem bark (35.0 g) was then partitioned with n – hexane to yield hexane fraction (HF). The aqueous portion was collected and partitioned with ethyl acetate (to yield ethyl acetate fraction (EAF). The aqueous fraction was equally partitioned with n – butanol to give butanol fraction (BF) and aqueous fraction (AqF). The fractions that were obtained were screened for in vitro antioxidant to establish their potency and used for the analyses.Phytochemical ScreeningChemical tests both qualitative and quantitative on the methanolic extracts and all the fractions of K. africana were doneusing standard procedures to identify the plant constituents as described by [15] [16] and [17]. Anti-Inflammatory StudiesMembrane Stability ActivityThered blood cell membrane stability activity assay was carried out using the method of [18].In vitro Antioxidant StudiesDetermination of Total Phenolic ContentThe total phenolic of the extract of K. africana was determined using the Folin–Ciocalteu’s method of [19] as described by [20].Determination of 1, 1-Diphenyl-2-Picrylhydrazyl Hydrate (DPPH) Radical Scavenging ActivityThe hydrogen or radical scavenging properties of the extracts was determined using the stable radical 1, 1–diphenyl-2-picrylhydrazyl hydrate (DPPH) according to the method of [21]and as described by [22]. Ferric Reducing Antioxidant Power Assay (FRAP)The FRAP assay used antioxidants as reductants in a redox linked colometric method with absorbance measured with spectrophotometer [23]. Total Antioxidant CapacityThe total antioxidant capacity of the test samples was determined according to the method of [24]. Sub-Chronic StudyThirty five (35) Wistar adult male rats weighing 200-300 g were randomly divided into 7 groups (A, B, C, D, E, F, and G) of 5 rats each. Group A was the control group and received Normal saline, group B was given arsenic trioxide dose only, group C was given 3 mg/kg body weight arsenic trioxide, and 300 mg/kg body weight ascorbic acid and served as positive control. Groups D and E were given 3 mg/kg body weight arsenic trioxide and different doses of 250 mg/kg and 500 mg/kg body weight of methanolic extract of K. africana stem bark respectively and group F and G were administered 3 mg/kg body weight of arsenic trioxide and ethyl acetate fraction of methanolic extract250 mg/kg and 500 mg/kg of K. africana stem-bark respectively. 2% Tween 20 was used as vehicular control. Route of administration was per oral using oro-gastric canula.Sacrificing of the Experimental AnimalsAt the end of the treatment, 24 hrs after the last dose, five rats from each group were sacrificed after 30 days of treatment by cervical dislocation. Blood samples were collected by ocular puncture at the two time intervals before sacrificing the animals and the sera were used for both biochemical and hormonal assays: testosterone, leuteinizing hormone (LH) and follicular stimulating hormone (FSH). At the two time intervals mentioned above, the testes were isolated from each rat, washed 3 times with potassium phosphate buffer (0.1 mol/L, pH 7.2), weighed and used to determine activity of the following testicular marker enzymes: Lactate dehydrogenase (LDH), acid phosphatase (ACP), alkaline phosphatase (ALP) and glucose-6- phosphate dehydrogenase. The testes, brain and other accessory sexual organs were also used for histological studies.The epididymis was also removed after sacrificing each rat at the two time intervals, cleaned from fats, weighed and used for evaluation of spermatozoa and histological study. Other accessory organs for reproduction (testes, seminal vesicles and prostate gland) were also isolated, weighed and used for histological study.Biochemical StudiesPreparation of Blood SerumFresh blood (5-10 ml) sample was collected at two time intervals mentioned above by ocular puncture from each rat into a clean plain labeled tube, allowed to clot, and then centrifuged at 3000 rpm for 10 min in a bench centrifuge (Model 800D, Micro field Instrument, England) at room temperature. The clear serum was separated and kept at -4°C till assay.Preparation of Testis HomogenatesThe testis was isolated from each rat at the two time interval, washed with potassium phosphate buffer and weighed. 0.5 g of testis was cut into small pieces and homogenized manually using pestle and mortal in 5 ml potassium phosphate buffer (testis/total buffer is 1:10). The supernatant was obtained after the homogenate was centrifuged at 6000 rpm for 5 minutes at 4°C using refrigerated centrifuge (TGL-16G, B-Bran Scientific and Instrument Company, England) and used as an enzyme extract for determination of the enzyme activities. Estimation of Biochemical Parameters Assay of Serum TestosteroneSerum testosterone was estimated as described by [25] using Randox kit. The assay is based on competitive binding technique.Assay of Serum Luteinizing Hormone (LH)Serum luteinizing hormone was estimated as described by [26] using Randox kit. The assay is based on competitive binding technique.Assay of Follicle Stimulating Hormone (FSH)Serum follicle stimulating hormone was estimated as described by [27] using Randox kit. The assay is based on competitive binding technique.Assay of Alkaline Phosphatase (ALP) ActivityThe testis homogenate alkaline phosphatase activity was estimated as described by [28] using Fortress kit.Assay of Acid Phosphatase (ACP) ActivityThe testis homogenate total acid phosphatate (ACP) activity was estimated as described by [28] using Fortress kit.Assay of Lactate Dehydrogenase (LDH) ActivityThe testis homogenate lactate dehydrogenase (LDH) activity was estimated as described by [29] using Randox kit.Extraction of Reduced Glutathione (GSH)A homogenate was prepared with 1.0 g of the liver sample with 10 ml of 250 mM Sucrose. The supernatant was then used for the estimation of reduced glutathione (GSH).Estimation of Glutathione Peroxidase (GPx) ActivitiesThe activity of Glutathione peroxidase (GPx) was determined according tothe method of [30].Determination of Superoxide Dismutase (SOD) ActivityThe level of SOD activity was determined by the method of [31]. Assay for Catalase ActivityCatalase activity was determined by using the direct UV assay method of [32].Protein DeterminationProtein determination was carried out according to the method of [33]. Sperm Evaluation StudiesCollection of Semen and AnalysisThe methods of collection used were as described by [34] and [35].Sperm Count and Motility AnalysisThe spermatozoa were counted by hemocytometer using improved Neubauer chamber as described by [36].Percentage Viability AssayLive/dead ratio was determined according to the method described by [37].Histological InvestigationThe histopathological analysis of the testes was carried out by standard procedure. The testes were fixed were sliced into approximately 0.3cm thickness and placed into 10% formosaline for fixation. The testes were dehydrated, mounted, sectioned and stained with hematoxylin and eosin. The slide were examined, captured with LEICA 750 light microscope interfaced with a Leica ICC50 light camera at the Department of Anatomy and Physiology, Obafemi Awolowo University, Ile Ife, Osun State.Statistical AnalysisAll data obtained were subjected to statistical analysis and the results were expressed as Mean ± SEM. Differences between mean values were determined by one way ANOVA using Graph Pad Prism (Graphical – Statistical package version 5) and comparisons were done by the Turkey test. Differences were considered to be significant if p<0.05.
3. Results
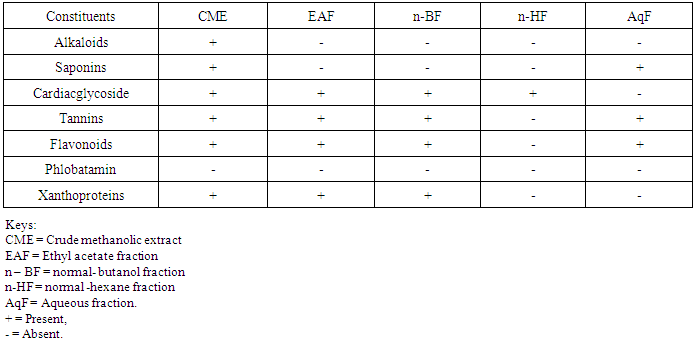
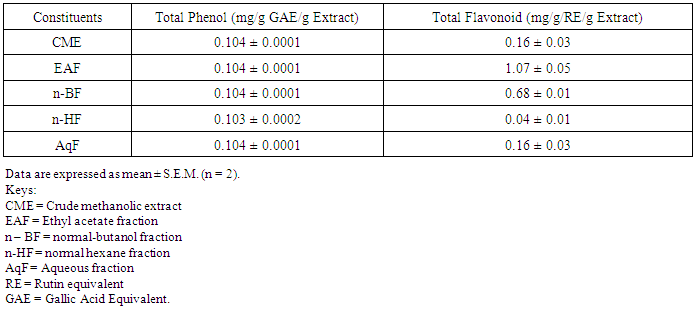
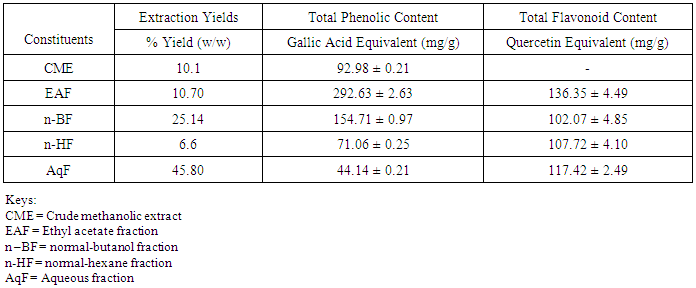
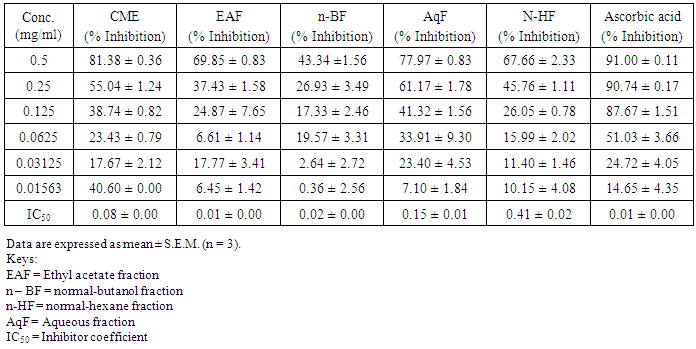
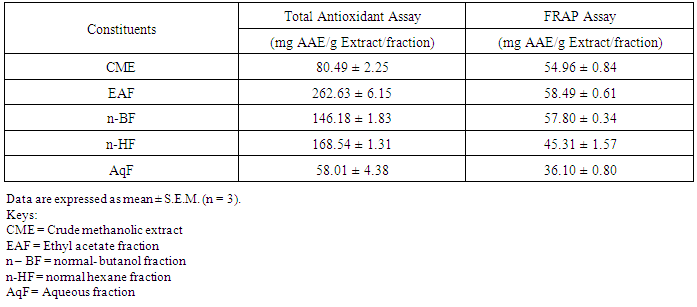
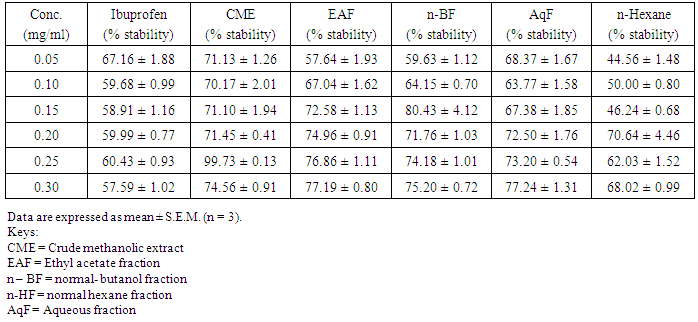
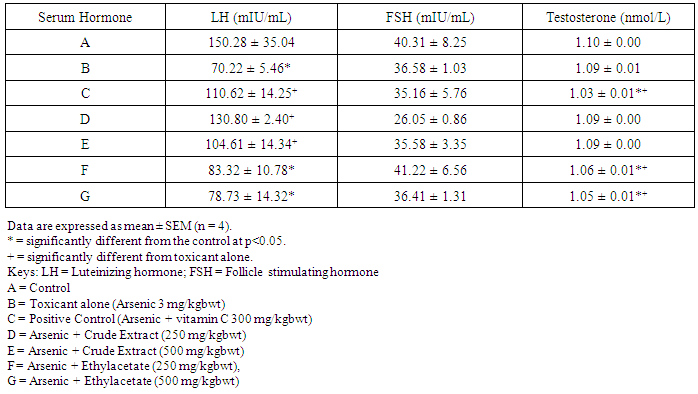
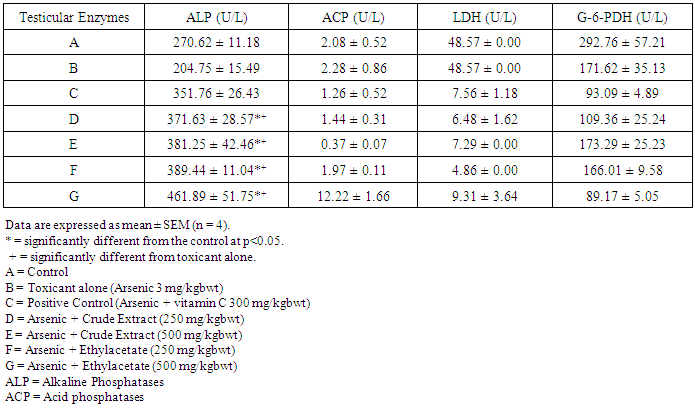
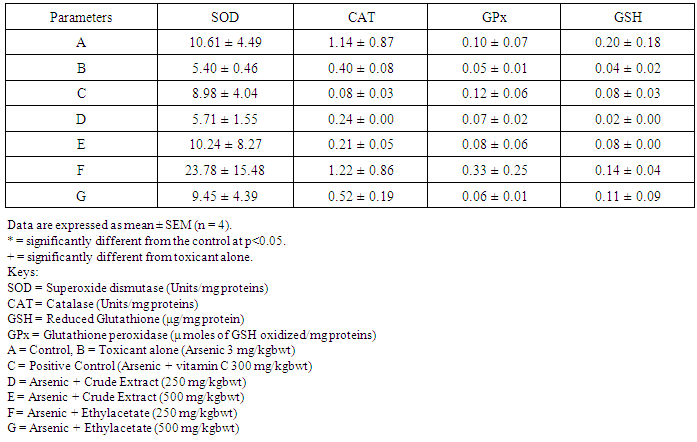
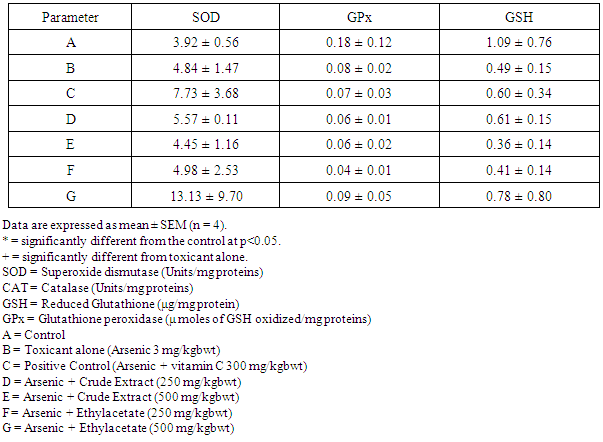
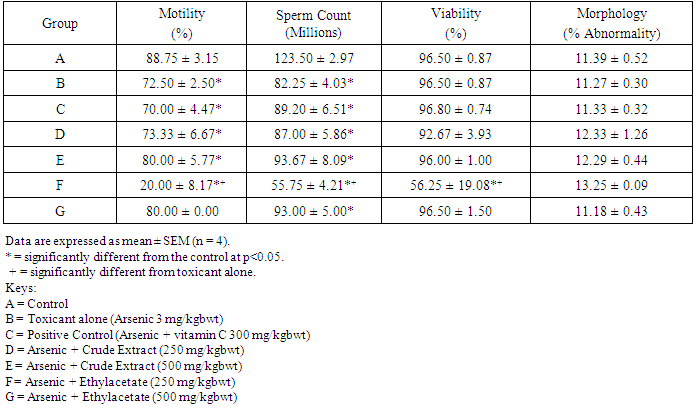
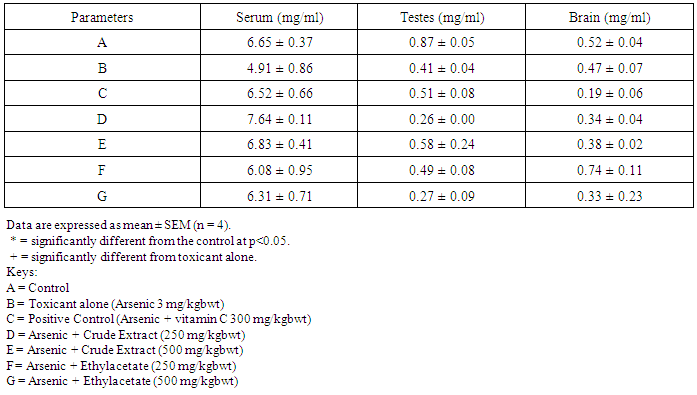
- Table 1 shows the results for the phytochemical constituents of the extract/fractions of K. africana stem bark, Table 2 shows the results for quantitative phytochemical screening of total phenol and flavonoids of the extract/fractions of K. africana stem bark, Table 3 shows the results for percentage yields and concentration of total phenolic content and total flavonoid constituents contained in K. africana stem bark, Table 4, shows the results for the 1,1-diphenyl-2-picrylhydrazyl hydrate (DPPH) radical scavenging activity of K. africana methanolic stem bark extract and its fractions, Table 5 shows the results for the total antioxidant assay and ferric reducing power (FRAP) activity, Table 6 shows the results for the membrane stability activity of crude methanolic extract of K. africana stem bark and its fractions on bovine red blood cells, Table 7 shows the results for the effects of arsenic and extract of K. africana on serum luteinizing hormone, follicle stimulating hormones, and testosterone hormone levels of male wistar albino rats, Table 8 shows the results for the effect of arsenic and extract of K. africana on testicular homogenate alkaline phosphatase, total acid phosphatase, lactate dehydrogenase and glucose-6-phosphatase dehydrogenase enzymes levels on male rats, Table 9 shows the results for the effect of K. africana stem bark extract on superoxide dismutase, catalase, glutathione peroxidase and reduced glutathione enzyme levels in the brain, Table 11 shows the results for the change in protein level in serum, testes and brain of arsenic-induced gonad toxicity treated with extract/fraction of K. africana stem bark, and Table 12 shows the results for the effect of arsenic and extract of K. africana on sperm count, sperm motility, live/dead ratio (LDR) and viability of male rats.
|
|
|
|
|
|
|
|
|
|
|
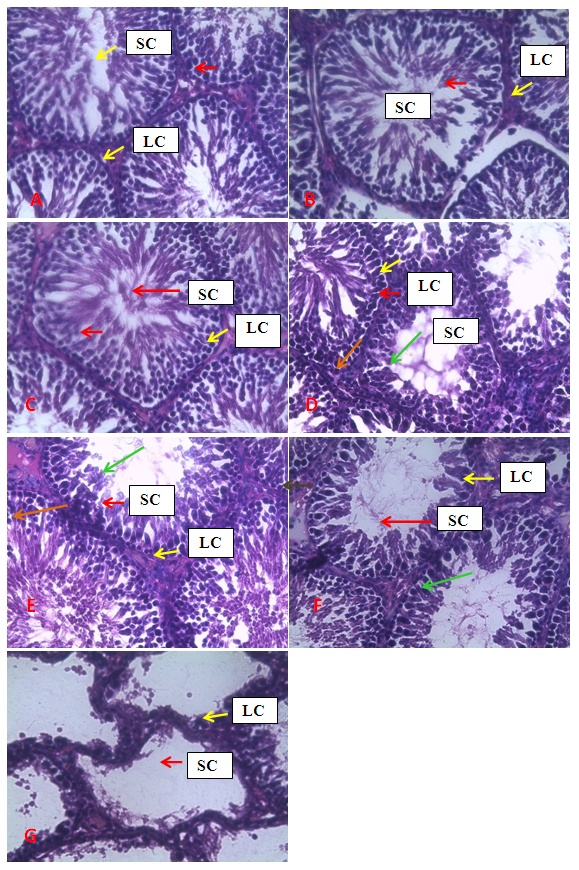 | Figure 1. Photomicrographs of the seminiferous tubules of groups A, B, C, D, E, F and G. showing normal spermatogenic (Red arrow) and Leydig (yellow arrow) cells in A and C. Degeneration of spermatids (Green arrow) is noted in D, E and F while whitish interstitial deposit (orange arrow) were noted in D and E. Patchy degeneration of the spermatogenic cells and interstitial splitting were noted in B. Complete degeneration of spermatogenic cells alongside distortion of general architecture of the testis were noted in G. Stain H&E. Mag. X400. LC = Leydig Cell, SC = Sertoli Cells |
4. Discussion
- Infertility and damage to the male gonad is a major clinical problem, affecting approximately 15% of all couples trying to achieve conception. This condition affectspeople medically and psychologically and the male factor is the sole or contributing factor in roughly half of these cases [38]. Of the many causes of male infertility; oxidative stress (OS) has been identified as one factor that affects fertility status and thus, has been extensively studied [38]. Spermatozoa, like any other aerobic cell, are constantly exposed to oxidative damage [39]. Oxygen is essential to sustain life as physiological levels of ROS are necessary to maintain normal cell function. Conversely, breakdown products of oxygen such as ROS can be detrimental to cell functions and survivalleading to oxidative stress and damage [40] [41]. Moderate increase in oxidative stress can activate cell growth which results in normal physiologic response. Conversely highly increased oxidative stress causes cell injury (e.g. DNA and protein damage, cell membrane disruption). Men with increased oxidative stress and DNA destruction have increased risk of fertility which can be more diagnosed with spermatozoa studies including sperm motility [41].Reactive oxygen species causes a decrease in sperm motility and thus increase the probability of infertility [42]. Exposure to arsenicals, which are used as herbicides, fungicides and rodenticides may cause soil, air and water pollution [43] and might be a factor considering the hormonal disruption that occurs with its use. Arsenical exposure through drinking water is common in many areas of the world, India Pakistan etc [44]. Metabolic disorders, hypertrophy of adrenal glands [45] and anemia [16]; inhibition of the activity of testicular steroidogenic enzymes [46] and reduction in the weight of the testis and accessory sex organs [47] are associated with exposure to arsenicals. A number of proteins and enzyme systems containing sulfhydryl group have been found to be altered by arsenic [45]. Arsenic effects mitochondrial enzymes and impairs tissue respiration, which seems to be related to the cellular toxicity [46].The present study focuses on evaluation of the gonado-protective potentials of K. africana (Lam.) Benth on arsenic-induced gonadotoxicity in male wistar rats. K. africana belongs to the family of Bignoniaceaes; commonly called the sausage tree because of its huge fruits [48] with many medicinal properties due to the presence of numerous secondary metabolites like saponins, steroids, tannins, flavonoids and alkaloids which are responsible for its antioxidant action against free radicals [49]. Phytochemicals are biologically active, naturally occurring chemical compounds found in plants, which provide health benefits for humans further than those attributed to macronutrients and micronutrients [50]. They protect plants from disease and damage and contribute to the plant’s color, aroma and flavor [51] [52]. In this study, the percentage yields obtained from the plant material were 10.1% .While the yields from other partitioned fractions were 6.6%, 10.70%, 25.14% and 45.80% for hexane, ethyl acetate fraction, butanol fraction and aqueous fraction respectively.The phytochemical screening of the crude methanolic extracts and fractions of K. africana stem bark showed the presence of secondary metabolites such as saponins, alkaloids, flavonoids, and cardiac glycosides; which is in agreement with the phytochemical screening reported on the same plant by [53]. Alkaloids, flavonoids, saponins, cardiac glycosides, tannins and xanthoproteins were founds in the crude methanolic extract of the K. africana; while the ethylacetate and butanol fractions showed the presence of flavonoids, tannins, cardiac glycosides and xanthoproteins respectively. The aqueous fraction showed the presence of saponins, tannins and flavonoids while the hexane fraction showed the presence of cardiac glycoside only. These phytochemicals are known to have bioactive activities in medicinal plants and may therefore be responsible for the antioxidant activities of theplant extracts. Tannins are generally known to be useful in the treatment of inflammed or ulcerated tissues and have remarkable activity in cancer prevention [54] [55]. Thus, the presence of these constituents in K. africana partly supports the common traditional use of the plant in the treatment of various diseases. Flavonoids have been shown to exhibit their actions through effects on membrane permeability, and by inhibition of membrane-bound enzymes such as the ATPase and phospholipase A2 and this property may possibly explain the mechanisms of anti-oxidative action of K. pinnata root extract [56].Severe techniques have been used to determine the antioxidant activity in vitro of the plant extracts in order to allow rapid screening of the fractions [57]. The antioxidant potential and screening of the methanolic extracts and all the fractions of K. africana were assessed and screened through diverse varieties of biological parameters and compared with the standard antioxidants. Due to the complex nature of phytochemicals; it was reported that the antioxidant activities of plant extracts cannot be evaluated by a single assay method; thus commonly accepted antioxidant of in vitro assay methods were adopted to evaluate the antioxidants potential of the plant extracts and its fractions. Polyphenols are pharmacologically active components of plants which are capable of neutralizing free radicals, chelating metal catalysts and inhibiting the activity of oxidizing enzymes in biological systems [58]. They are also capable of regenerating endogenous α-tocopherol in the phospholipid bilayer of the membrane to its active antioxidant form. This mechanism of antioxidant action confers health beneficial potentials on the polyphenolic compounds. The therapeutic potential of various medicinal plants have been attributed to their significant antioxidant potential due to the presence of phenolic compounds [59].Flavonoids are polyphenolic compounds with low molecular weight found in plant with diverse biological activities [60]. It has been reported that flavonoids displayed antioxidants potentials and their effects on human health cannot be over emphasized; their mechanism of action are through scavenging or chelating process [61]. In this study, ethyl acetate fraction of K. africana showed the highest total flavonoid content of 136.34 ± 4.5 mg QUE/g. It has also been established that flavonoids from medicinal plants possess high antioxidant potentials due to their hydroxyl groups and protect more efficiently against free radical related diseases like arteriosclerosis [62] [63]. Flavonoids are also involved in scavenging of oxygen derived free radicals [64]. Plant phenolic compounds are different in molecular structure, andare characterized by hydroxylated aromatic rings [65]. These compounds have been studied mainly for their properties against oxidative damage leading to various degenerative diseases, such as cardiovascular diseases, inflammation and cancer [66]. The results obtained from K. africana stem bark fractions showed that the total phenolics varied from 44.144 ± 0.211 mg GAE/g to 292.63 ± 2.633 mg GAE/g of fraction. The ethyl acetate fraction showed the highest total phenolics activity of 292.63 ± 2.633 mg GAE/g of fraction; indicating promising phenolic tendency; while the aqueous fractions showed the least total phenolics activity of 44.144 ± 0.211 mg GAE/g of fraction.In the DPPH assay, the activity was concentration dependent i.e. activity increased with increase in concentration. The IC50 values ranges from 0.01 - 0.42 mg/ml. The highest radical scavenging activity was showed by ethyl acetate with IC50 of 0.01 mg/ml. The strong inhibition displayed on DPPH radical could be linked topolyphenolic compounds which are capable of donating electrons or transferring hydrogen atom to neutralize free radicals and thus, could be a promising therapeutic agent to treat stress induced pathological conditions. This study indicates that the ethyl acetate fraction of the plant root has high antioxidant activity against DPPH than the hexane and methanol extract. It is due to the presence of high content of phenolic, which could be the most effective in protecting the body against various oxidative states.The reducing ability of a compound generally depends on the presence of reductants [67] which exhibits antioxidative potential by breaking the free-radical chain, donating a hydrogen atom [68]. Presence of reductant causes the conversion of the Fe3+/ferricyanide complex used in this method to the ferrous form. By measuring the formation of Perl’s Prussian blue at 700 nm, it is possible to determine the Fe2+ concentration. The results of the ferric reducing antioxidant power showed a concentration dependent change with ethyl acetate fraction having the highest activity of 58.5 ± 0.612 mg/AAE/g. This suggest that polyphenolic components within the ethyl acetate extract of K. africana play an important role in scavenging of free radicals, and the scavenging activity is increased with the increasing concentration of the plant extract. However, the total antioxidant assay of K. africana plant extract and their fractions showed a concentration dependent change. The ethyl acetate fraction showed highest total antioxidant activity of 262.63 ± 6.15 mg/AAE/g.Stabilization of red blood cells membrane was investigated to establish the mechanism of anti-inflammatory action of K. africana stem bark extract/fractions. The extract/fractions were effective in inhibiting the heat and hypotonic haemolysis at different concentrations. During inflammation, lysosomal hydrolytic enzymes are released into the sites which cause damage to the surrounding organelles and tissues with attendant variety of disorders [18]. It is well known that the viability of cells depends on the integrity of their membranes [69]. Exposure of red blood cells to injurious substances such as a hypotonic medium results in lysis of its membrane accompanied by haemolysis and oxidation of haemoglobin [70]. The haemolytic effect of hypotonic solution is related to excessive accumulation of fluid within the cell resulting in the rupture of its membrane. Such injury to RBC membrane will further render the cell more susceptible to secondary damage through free radical-induced lipid peroxidation [70] [69]. This view is consistent with the observation that the breakdown of bio-membranes leads to the formation of free radicals which in turn enhance cellular damage [71]. It is therefore expected that compounds with membrane - stabilizing properties, should offer significant protection to cell membrane against injurious substances [72] [71] [73]. Compounds with membrane-stabilizing properties are well known for their ability to interfere with the early phase of inflammatory reactions, namely the prevention of the release of phospholipases that triggers the formation of inflammatory mediators [74]. The results indicated that all the fractions contained principles that protected and stabilize the erythrocyte effectively. However, the crude methanolic extract exhibited and provided highest protection against induced lyses and all the fractions and the methanolic crude extract showed dose dependent membrane stabilizing activity across the concentration ranges. During inflammation, lysosomal hydrolytic enzymes are released into the sites of tissue damage which further cause damages to the surrounding organelles and tissues with attendant variety of disorders [75]. Such damage is undesirable because they inflict pains and cause discomfort. To arrest this anomaly, various methods were employed to screen and to study drugs, chemicals, herbal preparations that exhibit anti-inflammatory properties or potentials. Such techniques include uncoupling of oxidative phosphorylation (ATP biogenesis linked to respiration), inhibition of denaturation of protein, erythrocyte membrane stabilization, lysosomal membrane stabilization, fibrinolytic assays and platelet [76]. In this present study, stabilization of erythrocyte membranes exposed to both heat and hypotonic induced lyses were employed due to their simplicity and reproducibility. The activities of the membrane stabilization activity of the various fractions of the methanolic extract of K. africana were investigated and the results were compared with the standard anti-inflammatory drug Ibuprofen. All but the ethylacetate fraction demonstrated biphasic mode of protection implying that they do not have consist pattern of protection. Their modes of protection increased and decreased at the different concentrations tested. However, the mode of protection of the ethylacetate fraction was perfectly monophasic as the mode of protection increased with increase in concentration. Crude extract showed highest membrane stability of 99.73 ± 0.13% at 0.25 mg/ml and the least stability at 0.1 mg/ml with percentage stability of 70.17 ± 2.01%. The aqueous fraction had the highest activity of 77.24 ± 1.31% at 0.3 mg/ml. The ethylacetate which stood out from all other fractions for being the only monophasic fraction demonstrated its highest activity at 0.3 mg/ml with percentage stability of 77.19 ± 0.80%. The butanol fraction protected most at 0.15 mg/ml with percentage stability of 80.43 ± 4.12%; however as the concentration was increased further, it dropped down to 71.76 ± 1.03%. The hexane fraction showed the least protection of all the fractions evaluated. The highest percentage stability was 70.64 ± 4.46% at 0.20 mg/ml and at concentration above or below this was found to be toxic.The standard anti-inflammatory drug Ibuprofen demonstrated biphasic mode of protection at various concentrations tested. It demonstrated highest activity at lowest concentration (0.05 mg/ml) with percentage stability of 67.16 ± 1.88% but demonstrated signs of toxicity when the concentrations were increased. All the fractions including the crude extract of the K. africana demonstrated higher membrane stabilization potentials than the standard used and so, the plant should be explored for more anti-inflammatory properties. Therefore, the highest percentage stability of the crude extract could be due to the presence of certain phytochemicals such as saponins, cardiac glycosides, tannins and flavonoids which have been reported to possess profound membrane stabilizing activity on lyzosomal membrane. The tannins and saponins were reported to protect the erythrocyte membrane by binding to cations and other biological macromolecules [77]. The monophasic mode of protection of ethylacetate fraction on the other hand could be due to the presence of high concentrations of flavonoids and polyphenols which have been reported to possess strong anti-inflammatory and antioxidant activities mainly by inhibiting some of the free radical generating processes in the biological system.The study indicated that crude methanolic extract, aqueous and ethyl acetate fractions provided highest protection against induced lyses. The activities of the extract and fractions were higher than that of the standard drug, ibuprofen even at lower concentration ranges. The erythrocyte is analogous to the lysosomal membrane and its stabilization implies that the extract may stabilize the lysosomal membrane too. The stabilization of lysosomal membrane is crucial in reducing or limiting the inflammatory response by preventing the release of the activated neutrophil, which can cause tissue damage upon extra cellular release [77]. Majority of the non- steroidal drugs had been characterized for their possession of anti-inflammatory potency and activity. The broad therapeutic effects of flavonoids can be largely attributed to their antioxidant properties. In addition to an antioxidant effect, flavonoid compounds may exert protection against any disease through the inhibition of cyclooxygenase and lipoxygenase activities in platelets and macrophages [78].In this report, oral administration of arsenic caused a marked decrease in the levels of serum testosterone, FSH and LH levels. There was significant reduction in the serum hormonal levels in the toxicant group compared to the control, while there was slight increase in LH level in the extract/fraction treated groups up to the level in the control group. Remarkably, the level of both FSH and testosterone were significantly reduced at lower and higher doses of extract/fraction in the treated groups. This result is in accordance with a study conducted by [79]. Arsenic suppressed serum testosterone, FSH and LH along with testicular spermatogenesis indicating that arsenic acts at all levels of reproduction. FSH is the prime regulators of germ cell development. Testosterone was significantly decreased in all treated groups at lower and higher doses. This significant decrease in testosterone level may be as a result of direct damage of arsenic to leydig cells, which are the main sites of testicular androgen biosynthesis. Heavy metals are able to block or activate the steroid hormone receptors and/or to affect the level of sex hormones, thereby potentially affecting the development or growth of the male reproductive system [80]. The luteinizing hormone (LH) is glycoprotein released by the anterior pituitary; it stimulates testosterone production by leydig cells of the testes in males. Hypothalamic control of LH appears to be by a common releasing hormone; gonadoliberin (GnRH), with negative feedback control at the hypothalamic level by testosterone in the male [81]. The result revealed that exposure to arsenic (4 weeks) did not exert appreciable changes in LH and FSH levels in both lower and higher dose groups. There was an insignificant decrease in FSH and LH. This may cause changes in the pattern of the steroidogenic enzymes 3β- hydroxysteroid dehydrogenase and 17 β-hydroxysteroid dehydrogenase leading to inhibition of testicular androgen biosynthesis in adult rats, which is required for spermatogenesis in seminiferous tubules and sperm maturation in the epididymis [81]. A proposed mechanism that could explain arsenic induced toxicity, is blocking gonadotropin production and/or release by the pituitary, thereby testosterone by leydig cells is not stimulated, causing spermatogenesis arrest. This mechanism is supported by [82] who reported that the alteration of LH led to destruction of seminiferous epithelium and loss of germinal elements results in the reduction of the number of spermatids, sperm production in the testes as well as increase abnormality of sperms [83]. Reduced Glutathione (GSH) is one of the most abundant antioxidant in cells has been found to decrease during apoptosis [84]. Reduced glutathione has been hypothesized to play a role in the rescue of cells from apoptosis, by buffering an endogenously induced oxidative stress [85]. In this study, a decrease in reduced glutathione levels in the arsenic intoxicated animals may be responsible for enhanced lipid peroxidation via oxidative stress. But administration of test fraction of the plants increased the level of GSH, ameliorating the toxicity. Also, there was a decrease in the GSH activity in brain of arsenic treated in the study; showing that the brain cells may be at particular risk for oxidative stress. The brain derives its energy almost exclusively from oxidative metabolism through mitochondrial respiratory chain, and is relatively deficient in protective mechanisms compared to other tissues [86]. Arsenite interacts with thiol groups; thus, it can be directly toxic by blocking essential sulfhydryl groups of proteins and enzymes. This kind of binding can perturb the enzymes involved in carbohydrate metabolism and may alter the intracellular signaling mechanism [86]. Glutathione peroxidase an oxidative enzyme considered important in the decomposition of hydrogen peroxide, catalase decomposes hydrogen peroxides and protects the tissue from highly reactive hydroxyl radicals. The decreased activity of SOD, GPx and CAT in the tissues of arsenic-treated rats may be due to high concentration of free radicals generated by arsenic intoxication which may lead to decreased level or inactivation of these endogenous antioxidant enzymes. Treatment with extracts, vitamin C and fraction of K.africana significantly (p<0.05) increased the levels of SOD, GPx and CAT activities and thus, this result suggests that fraction ofK. africana contains free radical scavenging activity due to the presence of phenolic compounds, which could exert beneficial action against oxidative stress.Sperm count is one of the most sensitive tests for monitoring spermatogenesis and it is highly correlated with fertility. The result showed that there was reduction in the sperm count of all the treated groups. Histological structure of the testes appeared to confirm this, where it revealed degeneration and atrophy in some of the somniferous tubules associated with low luminal spermatozoa concentration. Also, there was reduction in the motility; the decrease in sperm motility after oral administration of arsenic may be due to androgen insufficiency which causes impairment in testicular functions by altering the activities of the enzymes responsible for spermatogenesis [87]. Abnormal sperm form percentage was significantly increased in all treated groups; showing effects of arsenic to somniferous tubules as shown by histological examinations of the testes of the treated rats.The testicular fluid contains both stimulatory factors as well as inhibitory factors that selectively alter the protein secretions [89]. Administration of arsenic in rats in this study led to reduction of protein concentration in testes, serum and brains of the animals compared to control. This reduction in protein synthesis might be responsible for the reduction in spermatogenesis [89]. But an appreciable high level of protein was seen with treatment with fractions of the K. africana. Thus it appears that the extract/fraction of K. africana has ability to restore protein synthesis.Sperm cell utilizes metabolic pathways for the production of energy. The decrease in alkaline phosphate activity indicated that arsenic treatment produced a state of decreased steroidogenesis where the intracellular transport will be reduced as the metabolic reactions to channelize the necessary inputs for steroidogenesis slowed down [90]. Acid phosphatases are enzymes capable of hydrolyzing orthophosphoric acid esters in an acid medium. Testicular acid phosphatase is up-regulated by androgens and is down-regulated by estrogen [91]. Activities of free lysosomal enzymes have been shown to rise when testicular steroidogenesis is increased. The results of the ACP assay in arsenic treated rats showed significant decrease in its activity in this study though not significant; but an elevated level was recorded in the fraction-treated rats. This supports previous studies that arsenic exposure leads to gonadotoxicity swith decreased activity of ALP and ACP [92]. Also, the result of this study showed significant decrease (p<0.05) in LDH activity in arsenic and K. africana treated groups. Testicular LDH is an essential component of the metabolic machinery of spermatozoa and is involved in the energy generation process for sperm motility [93]. The decrease in LDH activity implies a reduction in LDH activity in testicular tissues. The recorded decrease in LDH activity in arsenic treated rats probably indicates the interference of arsenic with the energy required for metabolism in testicular tissues. Arsenic substitutes phosphate intermediates, which could theoretically slow down the rate of metabolism and interrupt the production of energy [16]. G6PDH is used as an indicator of energy metabolism; it is involved in glucose metabolism and generates the cellular reductant, NADPH via the hexose monophosphate shut pathway (HMSP), which is utilized by glutathione reductase for the synthesis of glutathione. Thus the observed decreased in G6PDH levels in the treated groups may account for the significant reduction in GSH level due to reduced level of NADPH which are needed for glutathione synthesis.The decrease in changes in body weight and consequent increase in testicular organs weights seen in arsenic -induced control group was considered to be as a result of direct toxicity of arsenic and/or indirect toxicity that lead to damage. This indicates that, arsenic may have induced atrophy of the cells of these organs as well as elicit remarkable tissue damage [94] which may have led to the observed effects on the body and organ weights of these animals. However, all the induced treated groups experienced a significant increase in body weight changes as well as reduced change in organ weights, suggesting the possible curative effects of the fraction of K. africana against testicular gonads damage [95].The decrease in relative weight of the accessory sexual organs of the rats may be due to reduced tubule size, spermatogenic arrest and inhibition of steroid biosynthesis of leydig cells, which reflect the degree of degeneration caused by the toxicant; due to lower bioavalability of androgens [96].Histological study of the testes showed well arranged seminiferous tubules with distinct interstitial in control rats. The testicular tissue appears normal with full complement of spermatozoa concentration and spermatogenic cell line (spermatogonia, spermatocytes and spermatids). There was varying degree of cellular degeneration within the seminiferous tubules and interstitial in rats challenged with cadmium which was marked in rats treated with arsenic alone. There was also marked destruction of germ cells and seminiferous tubules in rats treated with arsenic and fraction of K. africana with little or partial restoration of the peripherial tissues. This damage was characterized by necrosis and destruction of the complete loss of the seminiferous tubules without any restoration, indicating the reduced ability of the testes to synthesize androgen necessary for spermatogenesis and spermiogenesis due to damage to the germ cells. The study concluded that treatment of rats with arsenic induces toxicity to the testes and impairs male reproduction, as it produces degenerative changes in the germ cells and inhibit androgen production acting primarily at the level of pituitary to inhibit the release of LH and FSH while the extract and fraction of K. africana at the doses considered did not have any protective effects on the testes. This may be due to short period between the time of administration and sacrifice of the animal; perhaps if more time had been allowed, there would have been more pronounced protective effect to the seminiferous tubules. Therefore K.africana may not likely be an agent in sexual dysfunction treatment.Though, the plant is used as an aphrodisiac in stimulating sexual desires and turgidity, cares must be taken; as it might have possibly negative effects on the male reproductive hormones and germ cell development. Further research is required to establish the exact bioactive compounds responsible for this activity and to shed more light on any demonstrated effects of K.africana extracts on other associated male reproductive organs like prostate gland and seminal vesicle, a target organ for androgen as this may assist in the elucidation of action against gonadal toxicity.
References
| [1] | Jensen, T.K., Bonde, J.P. and Joffe, M. (2006). The influence of occupational exposure on male reproductive function. Occupational Medicine.56: 544-553. |
| [2] | Jensen, T.K., Sobotka, T., Hansen, M.A., Pedersen, A.T., Lutz, W. and Skakkebaek, N.E. (2008). Declining trends in conception rates in recent birth cohorts of native Danish women. A possible role of deteriorating male reproductive health. International Journal of Andrology.31: 81-92. |
| [3] | Turgut, G., Abban, G., Turgut, S. and Take, G. (2003). Effect of overdose Zinc on mouse testis and its relation with sperm count and motility. Biology Trace Elements Research.96: 271-278. |
| [4] | Chowdhury, A.R. (2009). Recent advances in heavy metals induced effect on male reproductive function-A retrospective. Al Ameen Journal of Medical Science.2: 37-42. |
| [5] | Carlsen, E., Giwercman, N., Keiding, N. and Skakkebaek, N.E. (1992). Evidence for decreasing quality of semen during past 50 years. British Medical Journal.305: 609-613. |
| [6] | Traina, M.E., Ade, P., Siepi, G., Urbani, E. and Petrelli, M.G. (1994). A review of the effects of pesticide formulations on male fertility. International Journal of Environmental Health Research.4: 38-47. |
| [7] | Manna, P., Sinha, M., and Sil, P. C. (2008). Protection of arsenic-induced testicular oxidative stress by arjunolic acid. Redox Reproductive Journal.13: 67-77. |
| [8] | Carbone, P., Giordano, F., Nori, F., Mantovani, A. and Taruscio, D. (2007). The possible roles of endocrine disrupting chemicals in the aetiology of cryptorchidism and hypospadias: A population-based case control study in ruralsicily. International Journal of Andrology.30: 3-13. |
| [9] | Roy, P. and Saha, A. (2002). Metabolism and toxicity of arsenic: A human carcinogen. Current Science.82(1): 38-45. |
| [10] | Tariba, B., Kljaković-Gašpić, Z. and Pizent, A. (2011) Estimation of copper intake in moderate wine consumers in Croatia. Archives of Industrial Hygiene and Toxicology.62: 229-234. |
| [11] | Telišman, S. (1995) Interactions of essential and/or toxic metals and metalloid regarding interindividual differences in susceptibility to various toxicants and chronic diseases in men. Arh Hig Rada Toksikol. 46: 459-476. |
| [12] | Piasek, M. and Mikolić, A. (2009). Minerals and physiology (From essentiality to toxicity: a review of important minerals and their major impact on the human body’s physiology). In: Gašperlin L, Žlender B, editors. Role of Minerals in Food Technology and Nutrition. Ljubljana: Biotehniška fakulteta, Oddelek za živilstvo and Slovensko prehransko društvo. pp. 9-19. |
| [13] | Morton, W.E. and Dunnette, D.A. (1994). Health effects of environmental arsenic Arsenic. In: Arsenic in the Environment, Part II: Human Health and Ecosystem Effects, Nriagu, J.O. Ed. New York: John Wiley and Sons. pp. 17-34. |
| [14] | Sarkar, M., Chaudhuri, G. R., Chattopadhyay, A. and Biswas, N.M. (2003). Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian Journal of Andrology.1: 27-31. |
| [15] | Harborne, J.B. (1973). Phytochemical methods, Chapman and Hall, Limited, London. pp. 49-188. |
| [16] | Sofowora, E. A. (1993). Phytochemical Screening In: Medicinal plants and traditional medicine in Africa. Spectrum Books Ltd. Ibadan. Nigeria. |
| [17] | Trease, E. and Evans, W.C. (2002). Pharmacognosy.13th Edition. ELBS/Bailliere Tindall, London.pp. 229-246. |
| [18] | Oyedapo, O.O., Akinpelu, B.A., Akinwumi, K.F., Adeyinka, M.C. and Sipeolu, F.O. (2010). Red blood cell membrane stabilizing potential of extracts of Lantana camera and its fractions. International Journal of Plant Physiology and Biochhemistry.2: 46-51. |
| [19] | Singleton, V.L. and Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic phosphotungustic acid reagent. American Journal of Enology and viticulture.16: 144-158. |
| [20] | Gulcin, I., Oktay, M., Kirecci, E. and Kufreviolu, I. (2003). Screening of antioxidant and antimicrobial activities of anise (Pimpella anisum L.) seed extracts. Food Chemistry.83: 371-382. |
| [21] | Blois, M.S. (1958). Antioxidant determinations by the use of a stable free radical.Nature.181: 199-200. |
| [22] | Brace, J.L. (2001). SVfi inhibits reactive oxygen species generation and promote survival under conditions of oxidative stress in Saccharomyces cerevisiae. Yeast. 22(8): 641-652. |
| [23] | Benzie, I.F.F. and Strain, J.J. (1999). Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power. The FRAP assay. Analytical Biochemistry.239: 70-76. |
| [24] | Priesto, P., Pineda, M. and Aguilar, M. (1998). Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum Complex: Specific Applied to the determination of vitamin E. Analytical Biochemistry.269: 337-341. |
| [25] | Midgeley, J. (2001). Direct and indirect free testosterone assay methods and practice. Clinical Chemistry.47: 1353-1363. |
| [26] | Kosasa, T. S. (1981). Measurement of human luteinizing hormone. Journal of Reproductive Medicine.26: 201-206. |
| [27] | Wennink, J.M., Delemarre-van de Waal, H. A. and Schoemaker, R. (1990). Luteinizing hormone and follicle stimulating hormone secrection pattern in girls throughout puberty measured using highly sensitive immunoradiometric assay. Clinical.Endocrinology.33: 333-344. |
| [28] | Young, D.S., Thomas, D.W., Friedman, R.B. and Pestaner, L.C. (1972). Serum alkaline phosphatase: total activity and isoenzymes. Clinical Chemistry.18: 1041-1048. |
| [29] | Weisshaar, H.D., Gossrau, E. and Faderl, B. (1975). Normal ranges of alpha-HBDH, LDH, AP and LAP as measured with substrate optimated test charges. Medscape Women Health.26: 387-392. |
| [30] | Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G. and Hoekstra, W. G. (1973). Selenium biochemical role as a component of glutathione peroxidase. Journal of Science. 179: 588-590. |
| [31] | Mistra, H.P. and Fridovich, I. (1972). The role of superoxide anion in the autoxidation of Epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry. 247: 3170-3175. |
| [32] | Aebi, H. (1973). In methods of Enzymatic Analysis, Bergmeyer, H.U., Ed., Verlag Chemie Weinheim.pp. 673-684. |
| [33] | Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, K. J. (1951). Protein measurement with the Follin Phenol Reagent. Journal of Biology and Chemistry.193:265-275. |
| [34] | Akusu, M.O., Akpokodje, J.U., Ogwnegbu, S.O. and Oke, B.O. (1985). Difference in morphology of bull spermatozoa from normal and pathological testis during epididymal transit. Nigerian Veterinary Journal.14(2): 30-33. |
| [35] | Oyeyemi, M. O. and Ubiogoro, O. (2005). Spermatozoa and morphology characteristics in testicular and epididymal of large white boar in Nigeria. International Journal of Morphology. 23(3): 235-239. |
| [36] | Pant, N. and Strivastava, S.P. (2003). Testicular and Spermatotoxic effect of quinaphosn in rats. Journal of Applied Toxicology.23: 271-274. |
| [37] | Wells, M.E. and Awa, O.A. (1970). New technique for assessing acrosomal characteristics of spermatozoa. Journal of Diary Sciences.53: 227-232. |
| [38] | Sharlip, I.D., Jarow, J.P., Belker, A.M., Lipshultz, L.I., Sigman, M. and Thomas A.J. (2002). Best practice policies for male infertility. Fertility and Sterility.77: 873-882. |
| [39] | Sies, H. (1993) Strategies of antioxidant defence. European Journal of Biochemistry.215: 213–219 |
| [40] | De Lamirande, E. and Gagnon, C. (1995a). Capacitation-associated production of superoxide anion by human spermatozoa. Free Radical Biology and Medicine.18: 487-495. |
| [41] | De Lamirande, E. and Gagnon, C. (1995b). Increased production of intra- and extracellular superoxide anion by capacitating human spermatozoa. Journal of Andrology. 16(Supplement): P54. |
| [42] | Zare, S., Rahmani, Y., Eftekhaari, T.E., Faramarzi, A., Alavi, A. and Fallahi, S. (2013). Role of oxidative stress in male infertility. International Electronic Journal of Medicine (IEJM).2(2): 77-81. |
| [43] | Nickson, R., McArthur, J., Burges, W., Ahmed, K.M., Rarensero, F.P. and Rahman, M. (1998). Arsenic poisoning of Bangladesh ground water. Nature. 395: 338-345. |
| [44] | Chatterjee, A., Das, D. and Chatter, D. (1993). The study of underground water contamination by arsenic in the residential area of Behela and Calculta, due to industrial pollution. Journal of Biochemical Pharmacology.32(7): 1141-1148. |
| [45] | Biswas, N.M., RoyChowdhury, G. and Sarkar, M. (1994). Effect of sodium arsenite on adrenocortical activities of male rates: dose-duration dependent responses, Medicinal Science Research. 23: 1534-1540. |
| [46] | Sarkar, M., Chaudhuri, G. R., Chattopadhyay, A. and Biswas, N.M. (2003). Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. AsianJournal of Andrology.1: 27-31. |
| [47] | Sarkar, M., Ghosh, D. and Biswas, H.M. (1992). Effect of sodium arsenite on hematology in male albino rats. International Journal of Physiology and Allied Science.46: 116-120. |
| [48] | Sarkar, M., Biswas, N.M. and Ghosh, D. (1991). Effect of sodium arsenite on testicular and 3beta and 17beta hydroxysteroid dehydrogenase activation in albino rats: dose and duration dependent response. Medical Science Research. 19: 789-793. |
| [49] | Aiyelola, A.A. and Bello, O.A. (2006). Ethnobotanical potentials of common herbs in Nigeria: A case study of Enugu state. Educational Research and Review. 1(1): 16-22. |
| [50] | Santos, M.R., Rodriguez‐Gomez, M.J., Justino, G.C., Charro, N., Florencio, M. H. and Mira, L. (2008). Influence of the metabolic profile on the in vivo antioxidant activity of quercetin under a low dosage oral regimen in rats. British Journal of Pharmacology: Epub ahead of print. |
| [51] | Hasler, C. M. and Blumberg, J. B. (1999). Symposium on Phytochemicals: Biochemistry and Physiology. Journal of Nutrition.129: 756S-757S. |
| [52] | Gibson, E. L., Wardel, J. and Watts, C. J. (1998). Fruit and Vegetable Consumption, Nutritional Knowledgeand Beliefs in Mothers and Children. Appetite. 31: 205-228. |
| [53] | Marthai, K. (2000). Nutrition in the Adult Years. In Krause’s Food, Nutrition, and Diet Therapy, 10th Ed. Mahan, L. K. and Escott-Stump, S. Ed. American Cancer Society. 271: 274-275. |
| [54] | Yusuf, I., Arzai, A.H. and Haruna, M. (2012). Invitro susceptibility of amp c beta lactamase producers to extracts of Moringa oleifera, Cyperus articulatus and Kigelia africana. Global Journal of Science, Engineering and Technology.1: 19-26. |
| [55] | Motar, M.L.R., Thomas, G. and Barbosa Fillo, J.M. (1985). Effects of Anacardium occidentale stem bark extract on in vivoinflammatory models. Journal of Ethnopharmacy.95: 139-142. |
| [56] | Ruch, R.J., Cheng, S.J. and Klaunig, J.E. (1989). Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogens.10: 1003-1008. |
| [57] | Li, H., Wang, Z. and Liu, Y. (2003). Review in the studies on tannins activity of cancer prevention and anticancer. Zhong-Yao-Cai.26: 444-448. |
| [58] | Akanni, O.O., Owumi, S.E. and Adaramoye, O.A. (2014). In vitro studies to assess the antioxidative, radical scavenging and arginase inhibitory potentials of extracts from Artocarpus altilis, Ficus exasperate and Kigelia africana. Asian Pacicfic Journal of Tropical Biomedicines. 4(1): S492-S499. |
| [59] | Miliauskas, G., Venskutonis, P.R. and van Beek, T.A. (2004). Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry.85: 231-237. |
| [60] | Shahidi, F., Wanasundara, U.N. and Amarowicz, R. (1994). Natural antioxidant from low pungency mustard flour. Food Research International.27: 489-493. |
| [61] | Matés, J. M., Segura, J. A., Alonso, F. J. and Marquez, J. (2011). Anticancer antioxidant regulatory functions of phytochemicals. Current Medicinal Chemistry. 18: 2315-2338. |
| [62] | Kessler, M., Ubeaud, G. and Jung, L. (2003). Anti- and pro-oxidant activity of rutin and Quercetin derivatives. Journal Pharmacy and Pharmacology.55: 131-142. |
| [63] | Kris-Etherton, P. M., Hecker, K. D., Bonanome, A., Coval, S. M., Binkoski, A. E., Hupert, K. F., Griel, A. E. and Etherton, T. D. (2002). Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. American Journal of Medicine.113: 71-88. |
| [64] | Vaya, J., Mahmoud, S., Goldblum, A., Aviram, M., Volkova, N., Shaalan, A., Musa, R. and Tamir, S. (2003). Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy.Phytochemistry.62: 89-99. |
| [65] | Nijeveldt, R. J., Nord, E., Hoorn, E. C., Boelens, P. G., Norren, K. and Leeuwen, P. A. (2001). Flavonoids: a review of probable mechanisms of action and potential applications. American Journal of Clinical Nutrition.74: 418-425. |
| [66] | Balasundram, N., Sundram, K. and Saman, S. (2006). Phenolic Compounds in Plants and Agro industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chemistry. 99: 191- 203. |
| [67] | Matés, J. M., Segura, J. A., Alonso, F. J. and Marquez, J. (2009). Natural antioxidants: therapeutic prospects for cancer and neurological diseases. Mini Review in Medicinal Chemistry. 9: 1202-1214. |
| [68] | Duh, P. D., Tu, Y. Y. and Yen, G. C. (1999). Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat), Lebensmittel-Wissenschaft Und-Technologie. 32(5): 269–277. |
| [69] | Gordon, M. H. (1990). The mechanism of the antioxidant action in vitro, Food Antioxidants., Hudson, B. J. F., Ed., Elsevier Applied Science, London. pp. 1-18. |
| [70] | Ferrali, M., Signorni, C., Ciccoli, L. and Comporti, M. (1992). Iron release and membrane damage in erythrocytes exposed to oxidizing agents, phenylhydrazine, divicine and isouramil. Biochemical Journal.285: 295-301. |
| [71] | Augusto, O., Kunze, K.L. and Montellano, P.R. (1982). N-phenylprotoporphyrin formation in the haemogolobin-phenylhydrazine reaction. The Journal of Biological Chemistry.257: 6231-6241. |
| [72] | Maxwell, S.R.J. (1995). Prospects for the use of anti-oxidant therapies. Drugs.49: 345-361. |
| [73] | Liu, G. T., Zhang, T. M., Wang, B. E. and Wang, Y. W. (1992) Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochemical Pharmacology 43: 147-152. |
| [74] | Shinde, U.A., Phadke, A.S., Nair, A.M., Mungantiwar, A.A., Dikshit, V.J. and Saraf, V.O. (1999). Membrane stabilizing activity–a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia. 70: 251-257. |
| [75] | Aitadafoun, M., Mounieri, C., Heyman, S.F., Binistic, C., Bon, C. and Godhold, J. (1996). 4- Alkoxybenzamides as new potent phospholipase A2 inhibitors. Biochemical Pharmacology. 51: 737-742. |
| [76] | Sadique, J., Al-Rqobah, W.A., Buughaith, M. F. and El-Gundy, A. R. (1989). The Bioactivity of certain Medicinal Plants on the Stabilizing of RBC Membrane System. Fitoterapia.60: 525-532. |
| [77] | Oyedapo, O. O. and Famurewa, A.J. (1995). Anti-protease and membrane stabilizing activities of extracts of Fagara, Zanthoxyloides, Olaxsubscorpiodes and Tetrapleuratetraptera. International Journal of Pharmacognosy.33: 65-69. |
| [78] | Oyedapo, O. O., Akinpelu, B. A. and Orefuwa, S. O. (2004) Anti-inflammatory effect of Threobama cacao, root extracts. Tropical Medicinal Plants. 5(2): 161-165. |
| [79] | El-Shabrany, O.A., El-Gindi, O.D., Melek, F.R., Abdel-Khalik, S.M. and Haggig, M.M. (1997). Biological properties of saponins mixtures of Fagonia cretica and Fagonia mollis. Fitoterapia. LXVIII: 219-222. |
| [80] | Kumar, R., AliKhan, S., Pushpalatadubey, A.N., Singh, J.K., Ali, M. D. and Kumar, A. (2003). Effect of Arsenic Exposure in Testosterone Level and Spermatogonia of Mice.World Journal of Pharmaceutical Research. 2(5): 1524-1533. |
| [81] | Liu, X. F., Zhang, L. M., Zhang, Z., Liu, N., Xu, S. W. and Lin, H. J. (2013). Manganese-induced effects on testicular trace element levels and crucial hormonal parameters of Hyline cocks. Biological Trace Element Research. 151(2): 217-224. |
| [82] | Singh, S.K. and Pandey, R.S. (1990). Effect of subchronic endosulfan exposure on plasma gonadotrophins, testosterone, testicular testosterone and enzymes of androgen biosynthesis in rat. Indian Journal Experimental Biology.28: 953-956. |
| [83] | Mably, T.A., Bjerke, D.L. and Moore, R.W. (1992). In utero and lactation exposure of male rats to 2, 3, 7, 8 tetrachloro dibenzo-p-dioxin. Toxicology Applied and Pharmacology. 114: 118-126. |
| [84] | Caroll, R.S., Kowash, M. and Lofgren, J. A. (1991). In vivo regulation of FSH synthesis by inhibin and activin. Endocrinology. 129: 3299-3304. |
| [85] | Matés, J. M., Segura, J. A., Alonso, F. J. and Marquez, J. (2011). Anticancer antioxidant regulatory functions of phytochemicals. Current Medicinal Chemistry. 18: 2315-2338. |
| [86] | Fernandez, A., Kiefer, J. and Fosdick, L. (1995). Oxygen radical production and thiol depletion are required for Ca2+ -mediated endogenous endonuclease activation in apoptotic thymocyt. Journal of Immunology. 155: 5133-5139. |
| [87] | Zhang, T.L., Gao, Y.X., Lu, J.F. and Wang, K. (2000). Arsenite, arsenate and vanadate affect human erythrocyte membrane. Journal of Inorganic Biochemistry. 79: 195-203. |
| [88] | Sinha, N., Narayan, R. and Shanker, R. (1995). Endosulfan-induced biochemical changes in the testis of rats. Veterinary Human Toxicology. 37(6): 547-549. |
| [89] | Nair, A. and Verma, R.J. (2000). Effects of AFBI on the testes of mouse and amelioration by vitamine E. Indian Journal of Toxicology.7: 109-116. |
| [90] | Latchoumycandane, C., Chitra, K. C. and Mathur, P.P. (2002). The effect of methoxychlor on the epididymal antioxidant system of adult rats. Reproduction and Toxicology. 16(2): 161-172. |
| [91] | Yousef, G.M., Diamandis, M. and Jung, K. (2001). Molecular cloning of a novel human acid phoshatase gene that is highly expressed in the testes. Genomics. 74(3): 385-395. |
| [92] | Sadik, H. (2008). Effects of Dially Sulfide and Zinc on Testicular Steroidogenic in Cadmium-Treated Male Rats. Journal of Biochemistry and Molecular Toxicology. |
| [93] | Mollenhauer, H.H., Morre, D.J. and Rowe, L.D. (1990). Alteration of intracellular traffic by monensin: mechanism, specificity and relationship to toxicity. Biochemical Biophysics Acta.1031: 225-246. |
| [94] | Li, B., Li, X., Zhu, B. (2011). Sodium arsenite induced reactiveoxygen species generation, nuclear factor (erythroid-2 related) factor 2 activation, heme oxygenase-1 expression, and glutathioneelevation in Chang human hepatocytes. Environmental Toxicology. 13(4): 13-22. |
| [95] | White, M. (1999). Mediator of inflammation and inflammatory process. Journal of Allergy and Clinical Immunology. 103: 5378-5381. |
| [96] | Muyibi, S.A., Olorede, B.R., Onyeyili, P.A., Osunkwo, U.A., Muhammad, B.Y. and Ajagbonna, O.P. (2000). Haematological and histopathological changes of Casssia occidentalis leaf extract in rats. Nigerian Journal of National Products Medicine.4: 48-51. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML