-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2015; 5(3): 49-52
doi:10.5923/j.ajb.20150503.01
Determination of Antioxidants Activity in Tea Extract
Raghad Shakir Shayea Al-Obaidi, Dina Hamid Sahib
Department of pathological analyzes, Dijlah collage university, Baghdad, Iraq
Correspondence to: Dina Hamid Sahib, Department of pathological analyzes, Dijlah collage university, Baghdad, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The antioxidant activity of water extracts from white, green and black tea was measured using three methods. The highest antioxidant activity was found to be related to white tea. Hot water extracts of green and normal black tea showed also statistically significant antioxidant activities (P < 0.05). There was no statistically significant difference between the antioxidant activities of black tea and tea wastes, i.e. barks and old leaves discarded in the factory. In conclusion, the antioxidant capacities of water extracts of green tea leaves were almost comparable to similar extracts obtained from white tea, i.e. very young tea leaves together with blossoms. It was concluded that, although white and green tea are excellent sources of antioxidants, still old tea leaves and barks which are often considered as agricultural and factory wastes, can also be used as potential source of natural antioxidants.
Keywords: Tea, Antioxidant, Flavonoids, Polyphenols
Cite this paper: Raghad Shakir Shayea Al-Obaidi, Dina Hamid Sahib, Determination of Antioxidants Activity in Tea Extract, American Journal of Biochemistry, Vol. 5 No. 3, 2015, pp. 49-52. doi: 10.5923/j.ajb.20150503.01.
Article Outline
1. Introduction
- Tea extracts are powerful antioxidants due to the presence of chemical compounds such as epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epigallocatechin (EGC) and epicatechin (EC) as shown in Figure 1 [1]. Most of these compounds act as effective scavengers of free radicals [2]. It has been shown that tea, the common drink world wide, is able to reduce risk of coronary heart disease in aged men [3]. Many research works have focused on the natural antioxidants present in tea extracts. It has been reported that ethanolic extracts of tea strongly inhibit oxidation of canola oil [4]. Tea polyphenols are considered to be responsible for the anticarcinogenic, antimutagenic and protection against cardiovascular diseases of tea [5]. It has been shown that natural antioxidants in tea possess stronger antioxidative activity than synthetic antioxidants such as butylated hydroxyanisole, butylated hydroxytoluene and dl-α-tocopherol interestingly, tea polyphenols are much less toxic than that of butylated hydroxyanisole, butylated hydroxytoluene and dl-α-tocopherol [6]. However, the composition of various chemical compounds in tea may differ depending to growth conditions and region of production [7].
2. Materials and Methods
- Fresh green tea leaves and tea wastes (Camellia sinensis (L.) were collected from Diwaniya (Southern Iraq). The wastes were a mixture of old leaves comprised leaves barks and stems, whereas green tea was considered as the first 2-4 leaves and a bud. White tea was a Korean brand tea and purchased from a special supermarket in Baghdad. In order to inactivate polyphenol oxidase, all tea samples were soaked into boiling water for 3 min. They were then drained, dried at room temperature and ground to a fine powder. 2, 2-diphenyl-2-picrylhydrazyl hydrate (DPPH). 2,4,6-Tripyridyl-s-triazine (TPTZ), sodium acetate, ferric chloride, gallic acid, Folin-Ciocalteu’s phenol reagent and ferrous sulphate were purchased from Sigma represent tive.
2.1. Preparation of Methanolic Extract from Tea Leaves
- In a typical procedure, 10 g of the ground tea samples were boiled in 100 ml distilled water on a magnetic hot plate for 2 hours. The initial extracts were then re-extracted with 60 ml of distilled water at a temperature from 100 to 130°C for 30 minutes. The two fractions were the cooled to room temperature, filtered and combined. The extracted mixture was then dried 40°C and weighed to determine the yield [8].
2.2. DPPH Radical Scavenging Assay
- This method is based on the reduction of stable DPPH• when it accepts a hydrogen from an antioxidant compound. Radical scavenging activity of water extracts from white, green, black and tea factory wastes against stable DPPH° was determined spectrophotometrically. The changes in color (from deep-violet to light-yellow) were measured at 515 nm using a UV/visible light spectrophotometer (Ultrospec 3000 from Pharmacia Biotech). Briefly, each dry extract (0.025 g) was added to 10 ml of hot water. A freshly prepared solution of DPPH° in ethanol (6×10−5 M) was always used for UV measurements. 3 ml of ethanolic solution of DPPH• was then added to 77 μl extract solution in 1 cm path length disposable microcuvettes and mixed. The final mass ratio of extracts with DPPH° was approximately 3:1, 1.5:1, 0.75:1. The samples were kept in the dark for 15 min at room temperature and then the decrease in absorption was measured. Absorption of blank sample containing the same amount of ethanol and DPPH• solution was prepared and measured daily. The experiment was carried out in triplicate. Radical scavenging activity was calculated using the following relationship:% Inhibition = [ (AB – AA) /AB ] × 100Where: AB-absorption of blank sample (t = 0min); AA-absorption of extract solution (t = 15min).
2.3. Total Phenolic Content by the Folin–Ciocalteu Assay
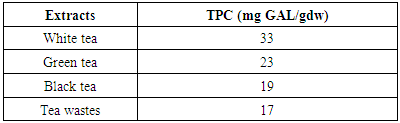
- The amount of total phenolic content (TPC) in aqueous tea extracts (100°C, with stirring) was determined according to the Folin-Ciocalteu procedure described in [9]. In short, an aliquot of 12.5 μl of water-soluble tea extract was mixed with 250 μl of 2% sodium carbonate solution in 96-well microplates and allowed to react for 5 min at room temperature. 12.5 μl of Folin-Ciocalteu phenol reagent (50%) was then added and allowed to stand for 30 min at room temperature before the absorbance of the reaction mixture was read at 650 nm using a plate reader. Calibration was achieved with an aqueous gallic acid solution (100–1000 μg/ml). Total phenol values were expressed as gallic acid equivalents (GAE) based on the calibration curve. The assay was carried out in triplicate for duplicate extractions giving six observations for each sample. Values given for each sample are means of six observations.
2.4. Evaluation of Antioxidant Activity Using FRAP Assay
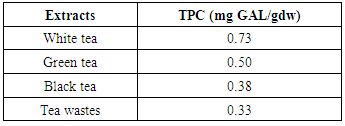
- The antioxidant activity (AOA) of water-soluble tea extracts (100°C, with stirring) was determined using the ferric reducing ability of plasma (FRAP) assay [10]. The working FRAP reagent was prepared by mixing 10 volumes of 300 mmol/l acetate buffer, pH 3.6, with 1 volume of 10 mmol/l 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mmol/l hydrochloric acid and with 1 volume of 20 mmol/l ferric chloride. Freshly prepared FRAP reagent (250 μl) was warmed to 37°C for 10 min, followed by addition of 8.5 μl extract and 25 μl deionized water. Sample absorbance was then read at 595 nm after 30 min in a plate reader. A standard curve was prepared using different concentrations (1-12 mmol/l) of FeSO4·7H2O. The results were corrected for dilution and expressed in mmol FeSO4/l. All solutions were used on the day of preparation. All determinations were performed in triplicate for duplicate extractions, giving six observations for each sample. Values for each sample are means of six observations.
2.5. Statistical Analysis
- All determinations were carried out in three triplicate and data were subjected to analysis of variance. Analysis of variance was performed using the ANOVA procedure. Statistical analyses were performed according to the MSTATC software. Significant differences between means were determined by Duncan’s multiple range tests. P values less than 0.05 were considered statistically significant.
3. Results and Discussion
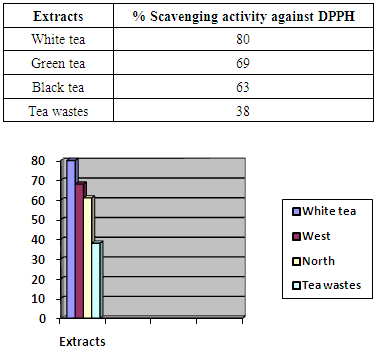
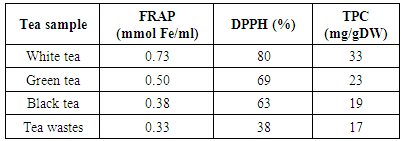
- The results obtained from DPPH assay are presented in table 1. The effect of fermentation could be explained by comparing the antioxidant activity of black tea with the two unfermented tea leaves, white and green. The wastes obtained from the tea factory were other parts of the plant such as barks and stems which had been fermented during the total fermentation process. Interestingly, these showed antioxidant activity similar to black tea.
|
|
|
|
4. Conclusions
- The results obtained from this study demonstrated that a considerable variation is present among both various tea samples determined by TPC as well as antioxidant properties expressed by FRAP and DPPH. The antioxidant activity of tea increased significantly (about two times) during various stages of fermentation when comparing white (unfermented) to black tea and black tea wastes (fully fermented). Interestingly, tea factory wastes showed antioxidant activity almost equal to that of black tea. Considering the very low price of wastes, the comparatively clean conditions they are processed and separated from the mail black tea and their significant antioxidant capacity, they could b considered for used in pharmaceutical applications.
ACKNOWLEDGEMENTS
- The authors express their sincere thanks to Dijlah collage university to bring all the efforts and support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML