-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2015; 5(2): 42-47
doi:10.5923/j.ajb.20150502.04
Improvement of Cocoyam Productivity with Effective Microorganisms (EM) and Indigenous Microorganisms (IMO) Manures
Mbouobda HD1, 2, Fotso1, 2, Tita M. A.1, Muyang RF1, 3, Omokolo ND2
1Department of Biology, Higher Teachers Training College (HTTC), University of Bamenda, Bamenda, Cameroon
2Laboratory of Plant Biology, Department of Biological Sciences, Ecole Normale Supérieure (ENS), University of Yaoundé 1, Yaoundé, Cameroon
3Department of Botany, Faculty of Science, University of Douala, Douala, Cameroon
Correspondence to: Mbouobda HD, Department of Biology, Higher Teachers Training College (HTTC), University of Bamenda, Bamenda, Cameroon.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Cocoyam (Xanthosoma sagittofolium (L) Schott) being one of the six most important root and tuber crop has received very little attention due to low productivity, and availability of planting materials. The intensification of agriculture has led to the exhaustment of natural resources and the use of inorganic fertilizers for mass production leading to environmental and sanitary impacts with the water beds being polluted with chemicals and the population intoxicated by pesticides. In order to develop natural fertilizer to access sustainable agriculture, two types of manure (EM commercial one and IMO local one which enrich the nutrient quality of soil with bacteria, fungi and cynobacteria) were used to improve the productivity of Xanthosoma sagittifolium (cocoyam) taking into account morphological and agronomic parameters as well as disease incidence and some biochemical analysis. A randomized complete block design (RCBD) with three treatments (EM manure, IMO manure and control) and six replications was used. From the results obtained there were significant differences (p < 0.05) in the height of plants and stem girth throughout the experiment in all the plants treated with EM manure followed by those treated with IMO manure. Plants treated with EM manure also had the highest weight of cormels while the control plants recorded the highest disease incidence (3.05 ± 1.93). The control plants produced the highest phenol content (143.89 ± 39.3 µg.g-1 FW) while peroxidase (POX) activity was highest in plants treated with IMO manure (8.99 ± 4.5 μg of CA of FW). Plants treated with EM manure had the highest pectin methyl esterase (PME) activity (5.48 ± 0.9 μg.mg-1 FW) and no significant difference was noticed between control plants and plants treated with IMO manure. Application of EM and IMO manure increased crop productivity, reduced disease resistance, and tolerance to adverse soil and climatic conditions.
Keywords: Manures, Agriculture, Productivity, Xanthosoma sagittifolium
Cite this paper: Mbouobda HD, Fotso, Tita M. A., Muyang RF, Omokolo ND, Improvement of Cocoyam Productivity with Effective Microorganisms (EM) and Indigenous Microorganisms (IMO) Manures, American Journal of Biochemistry, Vol. 5 No. 2, 2015, pp. 42-47. doi: 10.5923/j.ajb.20150502.04.
Article Outline
1. Introduction
- Cocoyam (Xanthosoma sagittifolium (L). Scott) is a staple root crop that belongs to the Araceae family. It is one of the six most important root and tuber crops worldwide [1]. Its importance is due to the fact that it produces storage starch corms, cormels; the leaves provide carbohydrates, proteins, fats and vitamins for humans [2]. It also provides direct income for farmers in many regions in the developing countries like Cameroon [3]. The soil is an indispensable resource that is essential for agriculture. Soil amendment with compost is an agricultural practice commonly used to improve soil quality and also to manage organic wastes for a sustainable land use [4]. Soil stability is a result of balance within its components. Presently, the negative effects of heavy applications of chemical inputs, in terms of production, environment, and quality deterioration are becoming apparent [5]. Microorganisms are very essential in improving soil health and fertility, therefore playing an important role in plant productivity. This makes them very important in maintaining soil fertility [6]. Microbial fertilizer makes use of beneficial microorganisms obtained from the soil and increase yield and quality of crops without a large investment of money and labour [7]. Moreover, microbial fertilizer can clean the environment and encourage the productive capacity of land by reducing the amount of chemical fertilizer consumption [7]. There is therefore need to apply such technology here in Cameroon in order to improve on our agricultural productivity and sustainability as it is productive, profitable, energy-conserving, environmentally-sound and less costly [8]. The aim of this study was to investigate the effects of EM and IMO manure on the growth and productivity of cocoyam. Specifically, the study was intended to; evaluate morphological and yield parameters of cocoyam grown under the treatments of EM and IMO manure, evaluating the effect of some enzyme activities (peroxidase and polyphenol oxidase) on cocoyam grown under the influence of EM and IMO and to compare the relationship between morphological, yield and biochemical parameters of the crops.
2. Materials and Methods
2.1. Location
- This research was carried out at the research farm of Higher Teachers’ Training College (HTTC) Bambili, in the University of Bamenda, Cameroon. Bambili has a total surface area of about 25.60 km2. It is located at latitude 5°99΄0΄΄North and longitude 10°15΄00΄΄East. It has an annual rainfall of 2000 to 3000mm. The topography of Bambili is hilly with steep slopes. Some area slope gently and these are used for planting tall trees and also for cattle grazing. Lowlands are used for crop cultivation and settlement [9].
2.2. Land Preparation
- The land was cleared and raked and ridges of 1 m by 3.5 m were formed. The experimental design used was the randomized complete block design (RCBD) with three treatments (EM, IMO and control) and six replications.
2.3. Preparation and Application of Manure
- IMO manure was prepared according to the method of Helen et al. (2006) [10] and EM manure was prepared according to the method of Higa (1991) [11]. 20 g of manure were applied separately one week before planting and 3 months after planting. Cormels of X. sagittifolium (100 to 125 g) with 3 to 4 buds were planted at a spacing of 1 m. Weeds were controlled manually and mulching was done at 3 months after planting.
2.4. Measurement of Morphological Parameters
- Plant height was measured at an interval of two weeks across five months using a measuring tape. The mean number of leaves for each ridge and treatment per crop was recorded.
2.5. Harvesting
- The cormels of cocoyam were harvested when the leaves were all yellow at 9 months after planting. They were weight and recorded.
2.6. Disease Incidence
- Disease incidence was evaluated by counting the number of leaves of infected plants showing yellowish brown lesions. This was done every month for 5 months.
2.7. Analysis of Total Phenol Content
- Fresh leaves of 0.5 g were ground at 4°C with 80% Methanol, incubated for 30 min and then centrifuged at 6000 g for 15 min. Total phenolic compounds were determined using Folin-Ciocalteu reagent according to the method of Macheix et al. (1990) [12]. Results were expressed in µg equivalent of chlorogenic acid (µg.g-1 CA of FW) by reference to the standard.
2.8. Protein Extraction and Analysis of POX and PME Activities
- 200 mg of fresh leaves were extracted with 3 ml Tris-maleate buffer (0.1M, pH 6.5) containing Triton X-100 (0.1 g.L-1), incubated for 1 h at 4°C and centrifuged at 6000 g for 15 min. The supernatant was used as the crude protein extract. POX activity was assayed spectrophotometrically at 470 nm using guaiacol as a substrate. 25 µL of enzyme extract was added to 2.5 ml of reaction mixture containing a solution of 1.5 M Tris-maleate buffer (pH 6.5) and 25 mM guaiacol. Reactions were initiated with 20 µl of H2O2 (10%) and ascorbic acid (0.25 mM) and stopped after 2 min. PME activity was determined at 610 nm, using a mixture of 2 ml of 0.1M pectin solution and 75 µl of extract. PME activity was expressed as enzymatic unit.mg -1of fresh weight (EU.mg-1 FW).
2.9. Data Analysis
- Data obtained were expressed as means ± SD and analyzed statistically using SPSS statistical software Version 17.0 (spss Inc., Chicago). Significant difference between mean values was determined by using analysis of variance (ANOVA). Duncan Multiple Range Test (DMRT) at 5% level of significance was used to compare means.
3. Results
3.1. Evaluation of Morphological Parameters, Yield and Disease Incidence
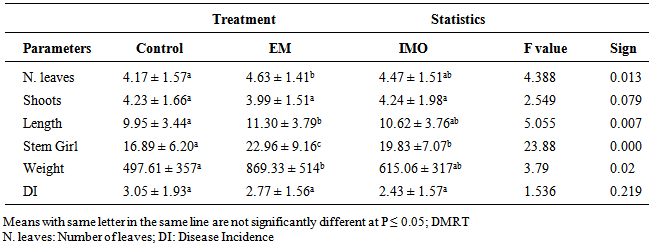
- The results obtained showed that plants treated with EM manure had the highest number of leaves (4.63 ± 1.41) followed by those treated with IMO manure. There was no significant difference in terms of number of shoots for all three treatments, with the plants treated with IMO manure having the highest number of shoots. Plants treated with EM manure had the highest stem length (11.30 ± 3.79 cm) followed by plants treated with IMO manure (10.62 ± 3.76 cm) and then the control plants. The stem girth was significantly higher for plants treated with EM manure (22.96 ± 9.16 cm) compared to those treated with IMO manure (19.83 ± 7.72 cm) and the control (16.89 ± 6.20 cm). As productivity, the weight of cormels produced by plants treated with EM manure (896.33 ± 541 g) was significantly different (P ≤ 0.05) from those of plants treated with IMO manure (615.05 ± 317.60 g) and the control (497.61 ± 357 g) (Table 1). From the results obtained, disease symptom was characterized by chlorotic leaves, wilting of the leaves at a tender age and premature decay of the roots. Disease incidence was not significantly different for all the treatments, with the control plot having the highest number of infected plants (3.05 ± 1.97) followed by plants treated with EM manure (2.77 ± 1.56) and then the plants treated with IMO manure (2.43 ± 1.57) (Table 1).
|
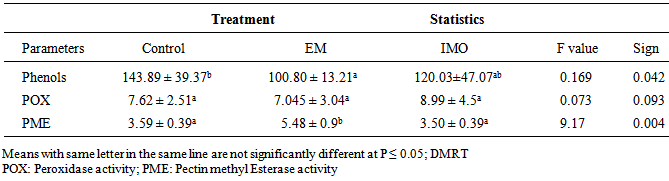
3.2. Evaluation of Phenol Contents and Enzyme Activities of the Different Treatments
- The results of the biochemical parameter showed that phenol content was significantly highest in the leaves of control plants (143.89±39.37 μg.g-1 of CA of FW) compared to plants treated with EM manure (100.80 ± 13.21 μg.g-1 of CA of FW). There was no significant difference in phenol content between both treated plants (EM and IMO manure) (Table 2). During the experiment, POX activity was highest in plants treated with IMO manure (8.99 ± 4.5 EU.g-1 FW) followed by the control plants (7.62 ± 2.51 EU.g-1 FW) and then the plants treated with EM manure (7.045 ± 3.04 EU.g-1 FW). However, there was no significant difference at P ≤ 0.05 for all the treatments.Plants treated with EM manure significantly had the highest PME activity (5.48 ± 0.9 EU.g-1 FW), and there was no significant difference in PME activity between control plants (3.59 ± 0.39 EU.g-1 FW) and plants treated with IMO manure (3.50 ± 0.39 EU.g-1 FW) (Table 2).
|
3.3. Correlation between Parameters Evaluated under Different Treatments
- The control plants had a negative and significant correlation (P ≤ 0.05) was noticed between POX and PME activities (-0.886*). Positive and significant correlations (P ≤ 0.01) were recorded between number of leaves and length of plants (0.589**), then stem girth (0.665**), and between length of plant and stem girth (0.595**) (Table 3).In plants treated with EM manure, no correlation was recorded between disease incidence and biochemical parameters (phenol content, POX and PME activities). However, there was positive and significant correlation (P ≤ 0.01) between number of leaves and length of plants (0.372**) and between weight of cormels and stem girth (0.559**) on the other hand (Table 3).Plants treated with IMO manure had a positive and significant correlation (P ≤ 0.05) was between POX activity and weight of cormels (0.829*), while a negative and significant correlation (P ≤ 0.01) was recorded between the same weight of plant and PME activity (-0.943). Positive and significant correlations (P ≤ 0.01) were noticed between number of leaves and length of plants (0.526**), then stem girth (0.453**) and between length of plants and stem girth (0.496**). The number of shoots was also correlated negatively with disease incidence (-0.285*) and with length of plants (-0.171*) at the level of significance of 0.05 (Table 3).
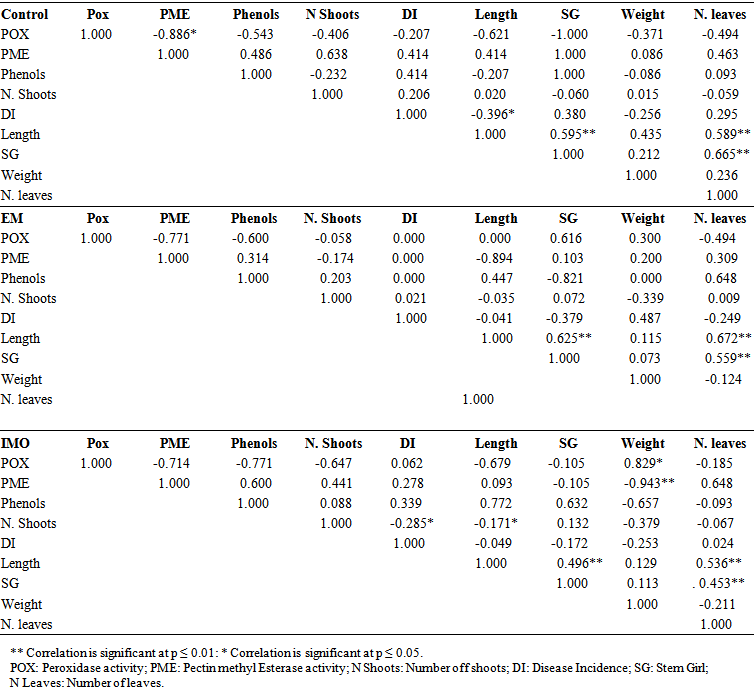 | Table 3. Rho-Spearman correlation between parameters studied under different treatments |
4. Discussion
- The aim of this study was to improve cocoyam productivity under EM and IMO manure application. From the results obtained, plants treated with EM manure had the highest number of leaves, stem girth, length of plants and productivity followed by plants treated with IMO manure. This could be due to the fact that application of manure increased maximum release of nutrients (Ca2+, Mg2+, N2, P, K+, and soil organic carbon) by the fertilizers [13]; which could lead to proper nutrition and thus increased photosynthetic activity of the plants leading to plant growth. The highest number of shoots recorded by plants treated with IMO manure could be due to the fact that, IMO manure contained bacteria that increased the rate of decomposition of the soil organic matter and associated increase in nutrient availability, thus increasing the number of shoots as obtained by Marulanda et al. (2009) [14]. No significant difference was obtained in terms of disease incidence, even-though control plants had the highest value. The presence of harmful microorganisms in the soil could lead to the stimulation of soil borne pathogens and consequently induction of plant diseases. Lower disease incidence recorded with both manure application could be related to the fact that beneficial microorganisms of these manures which contained enzymes like chitinase that enhance resistance to plants by the toxicant degradation could have reduced this incidence. There is an indirect mode of action of beneficial microorganisms where they suppress disease and insect attacks by stimulating increased production of plants natural defenses and suppression of soil-borne pathogens [15]. The higher yield obtained by EM manure followed by IMO manure is due to the fact that inoculation of EM culture to the soil improved growth, yield and quality crops, as well as enhanced physical and chemical properties [16]. Some beneficial fungal species also colonized plant roots and stimulated increased nutrient uptake which, in turn, improved the size and yield. EM also contains phytohormones and other biologically active substances that could delay senescence in plants [17].Phenol content was more important in control plants while POX and PME activities remained higher in Plants treated with EM followed by plants treated with IMO manures. Microorganisms in manure break down phenols into elements that contributed towards the mineralization of soil nitrogen and the formation of humus [18], thus contributing to the growth of the plant. The phenols chelate metals and improve soil porosity, providing active absorption sites and increasing the mobility and bioavailability of elements, (potassium, calcium, magnesium, copper, zinc, manganese, molybdenum, iron and boron), for plant roots [19].The highest level of POX exhibited by plants treated with both manures could be due to an increase in plant disease leading to an increase in disease incidence thus elevating peroxidase activity in diseased tissues. The role of peroxidase in plant is attributed to its ability to oxidize key metabolites like phenols in plants or pathogens [20]. Negative and significant correlation was noticed in control plants between POX and PME activities while in plants treated with EM manure; negative correlation was recorded between the weight of cormels and PME activity. PME facilitated demethylation of pectin by cross linking of pectin polymer chains and stabilizes the cell wall during expansion [21].Positive and significant correlations were recorded between morphological parameters in one hand and between some morphological parameters (stem girth) and weight of plants on the other hand for both manures application. Increase in some morphological parameters especially number of leaves might have resulted to better translocation of carbohydrate formation from the leaves to the tubers of cocoyam, thus influencing tuber production [22]. Similar results were obtained in taro (Colocasia esculenta) [23]; Irish potato (Solanum tuberosum) [24] and cocoyam (X. sagittifolium) [25]. In fact fermented mixed culture of naturally occurring species of microorganisms in manure such as photosynthetic and lactic acid bacteria, yeast, filamentous fungi and actinomycetes could improve yield, bulk and quality crops [16].
5. Conclusions
- The aim of this study was to investigate the effects EM and IMO manure on the growth and productivity of cocoyam while preventing environmental pollution from extensive application of inorganic fertilizers. From the results obtained, both EM and IMO manure increased yield of cocoyam. During this experiment, peroxidase and phenol content increased significantly, meaning that they could also be responsible for the growth and yield of cocoyam. Positive and significant correlations were noticed between morphological and agronomic parameters; even though disease incidence correlates negatively with morphological parameters of plants treated with IMO manure. Nevertheless, EM and IMO are added dimensions for optimizing our soil and crop management practices if properly used. Thus the local community should be sensitized on the use of EM which is inexpensive, to improve farming and thus help alleviate poverty.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML