-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2015; 5(2): 23-29
doi:10.5923/j.ajb.20150502.01
The Biochemical Effects of Cyanide on the Activity of the Transaminases and Alkaline Phosphatase in Broilers (Gallus domesticus L.)
Helen E. Kadiri, Samuel O. Asagba
Department of Biochemistry, Delta State University, Abraka, Nigeria
Correspondence to: Helen E. Kadiri, Department of Biochemistry, Delta State University, Abraka, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The biochemical effects of Cyanide (CN-) on the activity of transaminase enzyme and alkaline phosphatase in the domestic chicken (Gallus domesticus) given different doses (1, 2 and 3 mg kg-1 body weight of cyanide) directly (by gavage) and in the diet for different time period (4, 8 and 12 weeks) was investigated in this study. A total of eighty four-day old birds were used for this experiment. The chicks were divided into seven groups of twelve birds each: Group I- normal control, Group II, III and IV - received 1, 2 and 3 mg CN kg-1b.w. as Sodium cyanide (NaCN) directly respectively, while Group V, VI and VII received 1, 2 and 3 mg CN kg-1b.w. as NaCN in their feed respectively. The activities of the transaminases enzyme were determined by some well-established biochemical procedures in this study in order to ascertain and compare any impairment in the functions of the organs of birds given 1, 2 and 3 mg CN kg-1b.w directly and in their feed. The study revealed that Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) activities was significantly higher (p <0.05) in the serum and organs of birds given 2 and 3 mg CN kg-1b.w. when compared with the control in those given cyanide directly for each duration of exposure. While those that received CN- contaminated feed had significantly higher (p<0.05) activity of ALT and AST in the serum and organs of birds receiving 3 mg CNkg-1b.w. and significantly higher AST activity in the liver and kidney of birds receiving 2 mg CN kg-1b.w. at the end of 12 weeks of exposure. Alkaline phosphatase (ALP) activity was also significantly higher (p<0.05) in the liver and other organs of all treated birds given 3 mg CN kg-1b.w. directly and in feed. The findings indicate that the biochemical effect of CN- was more pronounced in birds exposed directly as compared to those exposed via feed. The findings also indicate that cyanide exposure altered enzyme levels in the organs of the birds, the increased activity of ALT and AST in the liver and other organs and corresponding increase in the serum of cyanide exposed chicks may be due to de novo synthesis and is a likely indication of increased amino acid metabolism, occasioned by increased energy demand. However increase in alkaline phosphatase activity in the organs and serum of birds, is an indication of animpaired liver function in birds given 3 mg CN kg-1b.w. directly and in feed with a greater impairment indicated in the birds given cyanide directly.
Keywords: Broilers, Cyanide, Transaminases, Alkaline phosphatase
Cite this paper: Helen E. Kadiri, Samuel O. Asagba, The Biochemical Effects of Cyanide on the Activity of the Transaminases and Alkaline Phosphatase in Broilers (Gallus domesticus L.), American Journal of Biochemistry, Vol. 5 No. 2, 2015, pp. 23-29. doi: 10.5923/j.ajb.20150502.01.
Article Outline
1. Introduction
- The absolute necessity of nutrition for continuance of life points to food as critical substance that must be maintained free from dangerous levels of toxicants. [1]. The high demand for cereals due to increasing human population and their use by millers for compounding livestock feeds coupled with the need for livestock products have led to the use of unconventional feeds for animal production [2]. These unconventional feed materials include sorghum, spent grains and wheat offal’s (by-product of sorghum and wheat malting respectively) as well as cassava [3], [4]. As a result of the increasing use of cassava in animal feeding there is greater exposure to dietary toxins from cyanogenic glycosides. It is generally accepted that the toxicity of cyanogenic glycosides is due entirely to the release of free cyanide [5]. The use of cassava in animal feed presents two major problems: the presence of cyanogenic glycosides in the tuber, and the remarkably low protein levels in fresh and dried cassava. [6], [7]. The goitrogenic effects of cassava are well documented [6], [8] and the release of cyanide could contribute to the detrimental effects which can occur when intake of this food is not held at a low level [8]. Many studies have reported the death of birds from cyanide poisoning through several routes, including exposure to cyanide salts or ingestion of cyanogenic plants [9] [10].Two types of cyanide toxicity have been recognized in man and animals: Acute toxicity and chronic toxicity. The mechanism of acute toxicity is well understood [10], [11], [12]. In animal tissues, cyanide forms a stable coordination complex with ferric ion and as a result tends to keep this metal in the higher oxidation state (Fe3+). This reduces the efficacy of iron as an electron carrier in the electron transport chain, thus inhibiting cellular respiration. This explains why acute cyanide toxicity symptoms depend upon the rate of release of free cyanide into the animal tissue [13], [14]. By contrast, the regular intake of small amount of cyanide in the diet does not result in death, but it is known to be responsible for the pathogenesis of several diseases, such as goitre [15] [14] renal problems [16] [17], [10], reproductive problems [17] and several neurological disorders [18] It has also been reported that the first sign of cyanide toxicity in birds appears between 0.5 and 5minutes after exposure and symptoms includes panting, eye blinking, salivation and lethargy. Breathing becomes laboured and intermittent, prior `to death [10].When certain types of cells are damaged, they may leak enzymes into the blood, where they can be measured as indicators of cell damage. Aspartate aminotransferase (AST), sometimes called serum glutamate oxaloacetate transaminase (SGOT) is one of such enzymes. It is found in many tissues including the heart, muscle, kidney, brain, and lung. The amount of AST in the blood is directly related to the extent of tissue damage [19]. Alanine aminotransferase (ALT), formally called serum glutamate pyruvate transaminase (SGPT) is produced mainly in the liver, and small amounts are found in the heart, muscle, and kidney. ALT catalyses’ the transfer of amino groups between L-alanine and glutamate to meet physiological needs [20]. Measurement of ALT or ALT and AST helps in the diagnosis of cardiac or liver disease. Although serum levels of both ALT and AST become elevated whenever disease processes affect liver cells, ALT is the more liver-specific enzyme [21]. Increase in the levels of ALT or AST or both may occur in situations of common bile duct stone e.g. transient biliary obstruction; medications e.g. acetaminophen; common liver disease causes e.g. alcohol abuse, cirrhosis, hepatotoxins, viral hepatitis, steatohepatitis (fatty liver); uncommon liver diseases e.g. autoimmune hepatitis.Alkaline phosphatase (ALP) is made mostly in the liver and by bone forming cells called osteoblasts with some made in the intestines and kidneys. The liver makes more ALP than other organs or bones and ALP test is used to detect liver or bone disorders. [22], [23] however there is a dearth of information on the ability of cyanide to influence the activities of these enzymes in birds. All these form the basis of this present study
2. Methods
2.1. Animal Procurement
- A total of eighty four one-day old birds purchased from Zartech farms Sapele, Delta state, Nigeria, were used for the study. The birds used in this study were maintained in accordance with the guidelines approved by the animal ethical committee, Delta state university Abraka, Delta State Nigeria. The chicks were kept in a standard wooden cage made up of wire gauze net and solid woods. The chicks were fed with starters mash for three weeks and thereafter, they were fed with growers mash, both mash were purchased from Top feeds, PLC, Sapele, Delta state, Nigeria. The chicks were also given tap water ad libitum
2.2. Experimental Design
- The chicks used for the experiment were divided into seven groups of twelve birds each; the groups were given the following treatments. The chicks were brooded on deep litter using 100 watt bulbs, flat plastic feeders and shallow drinkers for the first two (2) weeks of the experiment. The birds were fed starter mash experimental diets for four (4) weeks. Feed and water were provided ad-libitum. The birds were vaccinated against gumboro disease at the second and fourth weeks of age as first and second doses respectively. The experimental birds (Group II – IV) were intoxicated with cyanide every morning using gavage but they are fed with normal mash and tap water. Groups V-VII experimental birds were fed with different concentrations of cyanide in their feed every morning and normal tap water. The weights of the chicken were taken before administering the cyanide every morning. A third of the birds in each group were given this treatment for four weeks, while another third was for eight weeks. The final third in each group was treated for twelve weeks. Thus each group had four birds for each of the duration of exposure. The treatments are as shown belowGroup I – normal control.Group II – received 1 mg CN/kg body weight directlyGroup III – received 2 mg CN/ kg body weight directlyGroup IV – received 3 mg CN/kg body weight directlyGroup V – exposed to 1 mg CN/ kg feedGroup VI – exposed 2 mg CN/kg feedGroup VII – exposed 3 mg CN/kg feed
2.3. Collection of Samples
- After completing the duration specified for each sub group chicks were weighed and sacrificed under anaesthesia: The blood, sections of the digestive tracts, liver, brain, heart and kidney were collected.
2.4. Treatment of Samples
- The tissues of the digestive tract of the chicken were weighed and 20% homogenates were prepared using 10% sucrose solution. The homogenates were centrifuged and the supernatants obtained were used for biochemical analysis.Estimation of the activity of L-Aspartate aminotransferase (AST) and L-Alanine aminotransferase (ALT) activities in plasma and tissues were determined by the method of Reitman and Frankel [24].Estimation of alkaline phosphatase (ALP) was by the colorimetric method of Rec [25].
2.5. Statistical Analysis
- The results were expressed as Means ± Standard Deviation. Levels of significance between groups were assessed using student t-test analysis. While those with a significant difference were further analysed using Kruskal-Wallis Non-Parametric ANOVA Test to know the groups that causes the significant differences. A P-value of 0.05 was considered as statistically significant.
3. Results and Discussion
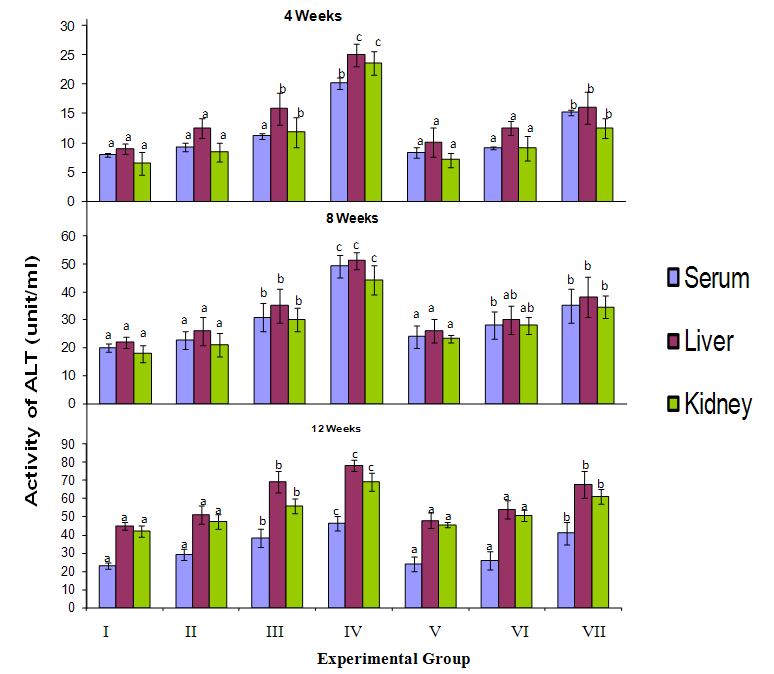
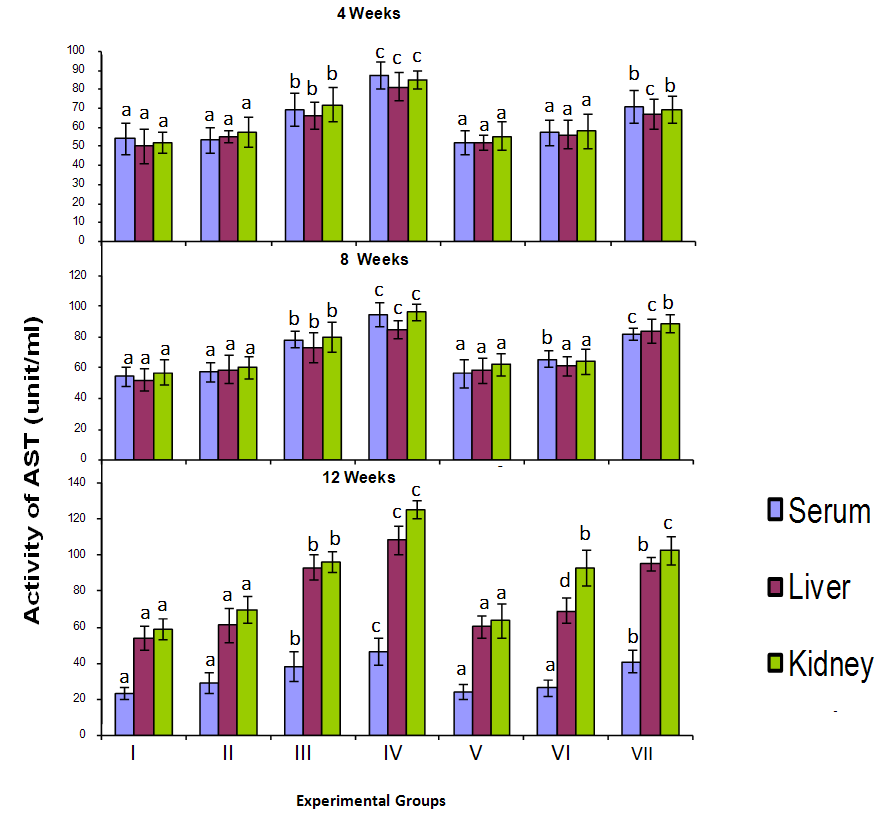
3.1. Effect on L–Aspartate Aminotransferase (AST) and L-Alanine Aminotransferase (ALT)
- The activities of Alanine aminotransferase (ALT) and Aspartate Aminotransferase (AST) in the serum, liver and kidney of birds exposed to cyanide are shown in Figure 1. Serum ALT and AST activities in birds given 3mg CN/kg body weight directly (group IV) was significantly higher (p<0.05) than that of those birds given lower doses (groups II and III) after 4 weeks of exposure. The same is true for birds exposed to cyanide for 8 and 12 weeks. Similarly the level of serum ALT and AST activity in birds given 3mg CN tainted food (group VII) was significantly higher (p<0.05) than those of birds fed lower cyanide diet for each of the exposure interval. However the serum ALT and AST activity of birds exposed to cyanide food was lower than that of birds given corresponding levels of cyanide directly.
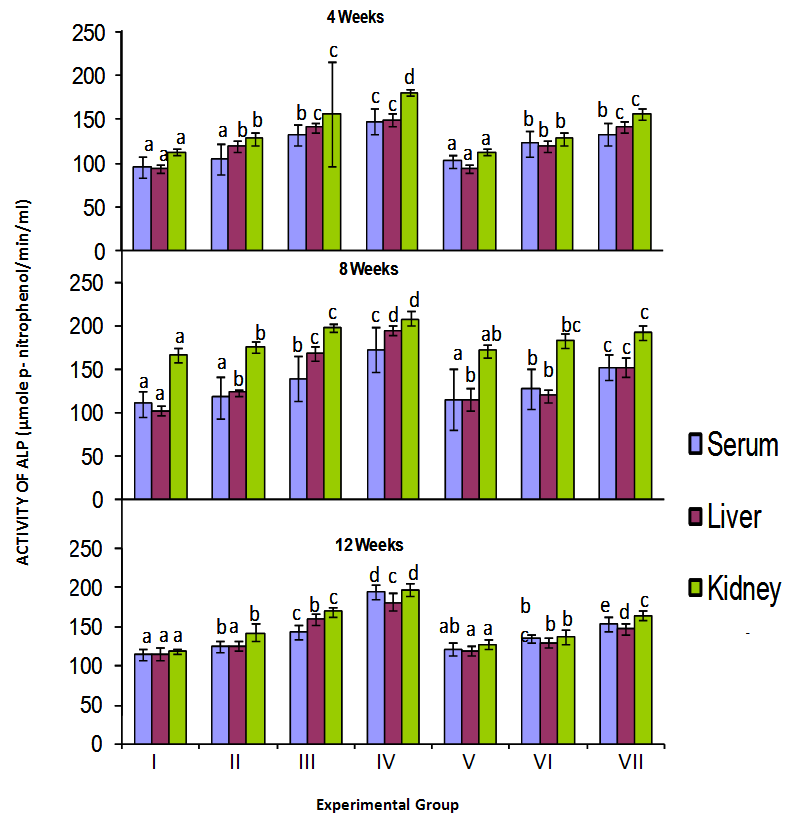
3.2. Effect of Cyanide on Alkaline Phosphatase
- The activities of alkaline phosphatase (ALP) in the serum, liver and kidney of birds exposed to cyanide are shown in Figure 3. There was a dose dependent increase in serum ALP activity in birds exposed to cyanide directly which was particularly significant in groups III and IV birds relative to control for each of the exposure periods. Similarly, there was significant increase in serum ALP activity in birds exposed to cyanide contaminated food as the amount of cyanide increased for all the exposure periods. It was also observed that increase in serum ALP activity was higher in birds given cyanide directly as compared to those exposed to cyanide via food for each of the exposure periods. The data in figure 3 also indicates significantly higher (p<0.05) ALP activities in the liver and kidney of treated birds when compared with the control (group V excluded) of each exposure period. Like in the serum, ALP activities in the organs of birds given higher doses of cyanide directly and via food were significantly increased in a dose dependent manner for each period of exposure. Also, the ALP activities of organs of birds fed cyanide tainted feed were lower than that of those treated with corresponding level of cyanide directly. In addition the highest activities of ALP were observed in the kidney of birds when compared to the liver in each period of exposure to cyanide.
4. Conclusions
- The results of this research work shows that the biochemical effects of cyanide on the activities of the transaminases and alkaline phosphatase was more pronounced in birds exposed directly to cyanide when compared to those exposed via feed. The findings also indicate that cyanide exposure altered enzyme levels in the organs of the birds, the increased activity of ALT and AST in the liver and other organs and corresponding increase in the serum of cyanide exposed chicks may be due to de novo synthesis and is a likely indication of increased amino acid metabolism, occasioned by increased energy demand.While the high activity of alkaline phosphatase in the serum and liver of birds is an indication of impairment of liver function however although a similar pattern was observed in the kidney, further studies will have to be carried out to ascertain if there is any impairment to kidney function.
ACKNOWLEDGEMENTS
- I wish to appreciate Tetfund for sponsoring this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML