-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2015; 5(1): 6-14
doi:10.5923/j.ajb.20150501.02
Severe Hepatotoxicity and Nephrotoxicity of Gasoline (Petrol) on Some Biochemical Parameters in Wistar Male Albino Rats
1Department of Science Laboratory Technology (Biochemistry Unit), School of Technology, Lagos State Polytechnic, Lagos, Nigeria
2Department of Science Laboratory Technology (Chemistry Unit), School of Technology, Lagos State Polytechnic, Lagos, Nigeria
Correspondence to: Momoh J., Department of Science Laboratory Technology (Biochemistry Unit), School of Technology, Lagos State Polytechnic, Lagos, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The present study was carried out to determine the severe hepatotoxicity and nephrotoxicity of gasoline on liver, kidney, hematological and oxidative stress parameters in wistar male albino rats. Preliminary toxicity study to determine the volume of gasoline that could cause toxicity was carried out using 56 healthy male albino rats. The calculated LD50 is 0.75ml of gasoline per kg body weight of the animals.Another set of 20 male albino rats were grouped into two groups and used for the biochemical analysis: Group A animals were the control group and Group B animals were administered with 0.5ml of gasoline per kg body weight. Preliminary toxicity study shows that group B rats exhibited changes in behavioural pattern such as restlessness, salivation, coma, respiratory distress, sedation and death. The results of this study shows that WBC values were significantly increased (P<0.05) in group B animals compared to group A animals. The result of this study shows that HGB, RBC and HCT values were significantly reduced (P<0.05) in the group administered with gasoline (group B) compared to the healthy group (group A). All the plasma liver biomarker enzymes: AST, ALT, ALP and GGT were significantly increased (P<0.05) in group B rats compared to group A rats. This is an indication of impaired liver function. The plasma total bilirubim value increased in group B rats while their plasma total protein values significantly reduced (P<0.05) when compared to group A rats. The liver histopathological examination also confirmed that administration of gasoline caused liver inflammation and cirrhosis in group B animals. Administration of gasoline to group B animals caused significant reduction (P<0.05) in the activities of SOD, CAT and GSH values of the liver homogenate. The MDA values were significantly high in group B rats compared to group A rats. This signifies the existence of hepatic lipid peroxidation and oxidative stress in group B animals.
Keywords: Hepatotoxicity, Nephrotoxicity, Gasoline, Histopathology, Hematological and Oxidative stress parameters
Cite this paper: Momoh J., Oshin T. T., Severe Hepatotoxicity and Nephrotoxicity of Gasoline (Petrol) on Some Biochemical Parameters in Wistar Male Albino Rats, American Journal of Biochemistry, Vol. 5 No. 1, 2015, pp. 6-14. doi: 10.5923/j.ajb.20150501.02.
Article Outline
1. Introduction
- Crude oil exploration is the major income of the Nigerian economy and constitutes about 90% foreign exchange earning of the nation. Berepubo et al. [1] and Carls et al. [2] have shown that various environmental pollutants, particularly those associated with crude oil cause many biochemical and toxic effects in terrestrial and marine animals. Crude petroleum is a mixture of different hydrocarbons and metals [3]. The chemical composition of crude petroleum varies between geographical locations. Crude oil is refined into fractions of petrol, kerosene, diesel, heavy gas oils, lubricating oils, as well as residual and heavy fuels, among others. However petrol, kerosene and diesel are the most commonly used fractionated crude petroleum products. Petrol contains aliphatic, aromatic and a variety of other branched saturated and unsaturated hydrocarbons [4, 5]. Gasoline is used as fuel for motor vehicles, generators and power plants. It is also used as pesticides and cleaning agent. Gasoline contains over 500 hydrocarbons that may have between 3 to 12 carbons per molecule. Previous studies have reported the hematotoxic [6] and hepatotoxic, [7, 8] effects of hydrocarbons. Exposure to various fractionated products of crude petroleum has been reported to cause impairment of renal function evident by the derangement of serum electrolytes. [7, 9, 10]. Some of the biomarkers used to determine hepatotoxicity levels include: alkaline and aspartate aminotransferase, Carboxylesterase, lactate dehydrogenase; as well as alkaline and acid phosphatase [11, 12]. Oxidative stress is an in balance between oxidant and antioxidants in favour of the oxidant leading to cell, tissue and organ damage. Oxidative stress produces reactive oxygen species (ROS) in excess of the available antioxidant buffering capacity [13]. Metabolism of poisonous chemical substances takes place in the liver, which accounts for the organ’s susceptibility to metabolic induced hepatotoxicity. The crude oil and its products generate free radicals and promote a variety of chemical reactions, such as depletion of reduced glutathiones or inducing lipid peroxidation. Sudakov, [14] Showed that increased levels of lipid peroxidation are indicative of severe liver, kidney and heart damage such as acute myocardial infarction and artherosclerotic plaque stability. Crude oil organic compounds are removed as solid wastes from the oil refinery and are referred to as sludge. Generally, the crude oil sludge and other waste products are disposed as landfills which create some health and environmental hazards [15]. This study was designed to investigate the biochemical and toxicological effect of gasoline on liver, kidney, hematological and oxidative stress parameters in Wistar male albino rats.
2. Materials and Methods
2.1. Analysis of the Gasoline (Petrol) Used for the Analysis
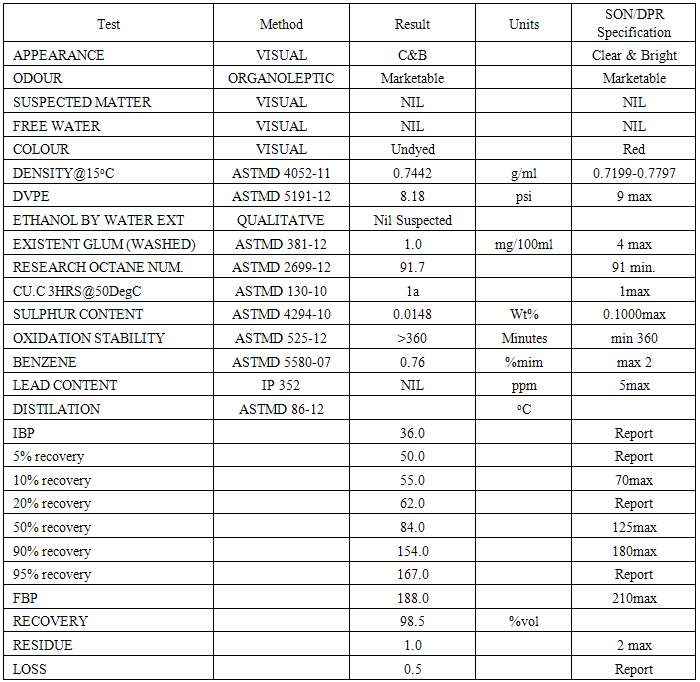
- The gasoline were bought from Total Nigeria PLC and analysed in SGS Inspection Service Nigeria Limited to ascertain it to be gasoline with the SON/Department of petroleum product (DPR) Specification using American Society for Testing and Material (ASTM) Methods.
2.2. Experimental Animals
- Male wistar albino rats with body weight ranging from 150 to 200g were obtained from Nigeria Institute of Medical Research (NIMR), Lagos, Nigeria. They were acclimatized for one week to Laboratory condition of 23 ± 2 oC. They were kept in plastic cages and fed with commercial rat chow and supply with water ad-libitum. The rats were used in accordance with NIH Guide for the care and use of laboratory animals; NIH Publication revised (1985) NIPRD Standard Operation Procedures (SOPs).
2.3. Preliminary Toxicity Study
- Preliminary toxicity study to determine the volume of petrol that could cause toxicity and death was carried out using 56 male albino rats. The rats were divided into seven groups of eight rats per group and were administered orally separately with 0.5, 1, 1.5, 2, 2.5 and 3ml of gasoline per kg body weight of the animals respectively. The seventh group was given water ad-libitum. The rats were observed over 72 hour’s period for nervousness, salivation, stretching, dullness, in-coordination and death. The LD50 (Median Lethal dose) was calculated. From the range of doses administered, 0.5ml of petrol per kg body weight of animals was chosen for this study.
2.4. Grouping of Experimental Animals
- Based on the results of the preliminary toxicity study, another set of 20 healthy male albino rats were grouped into two groups of ten rats per group as shown below: Group A – control group (healthy animals). Group B – animals administered with 0.5 ml/kg body weight of petrol.
2.5. Collection of Blood Samples
- The albino rats were sacrificed by cervical decapitation after 24 hours fasting on the eighth day. Blood were collected from the male albino rats by ocular puncture into EDTA tubes for hematological analysis and the remaining blood were collected in an heparinised bottle and centrifuge at 3000 rpm for 20 minutes using a centrifuge and the plasma stored at -20°C.
2.6. Determination of Hematological Parameters
- The hemoglobin concentration (HGB), total red blood cell (RBC), white blood cell count (WBC), Hematocrit (HCT), and other hematological parameters were determined in the blood using BC-3200 Auto Hematology Analyzer in University of Lagos Teaching Hospitals (LUTH) in Idi-araba, Lagos, Nigeria.
2.7. Estimation of Liver Biomarker Enzymes
- Plasma enzymes like alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gamma glutamyltransferase (GGT) were determined using Randox diagnostic kits. The total protein (TP) and total bilirubin (TB) were also determined using Randox diagnostic kits.
2.8. Determination of Kidney Function Test
- Plasma uric acid (UA), urea (UR) and creatinine (CR) values were determined using Randox diagnostic kits.
2.9. Histopathological Studies
- The histopathological analyses were assayed in the Department of Anatomy, college of Medicine, University of Lagos, Idi-Araba, Surulere, Lagos, Nigeria. Abdomens were opened to remove the liver. Some of the livers were fixed in Boucin’s solution (mixture of 75 ml of saturated picric acid, 25 ml of 40% formaldehyde and 5ml of glacial acetic acid) for 12 hours, then embedded in paraffin using conventional methods [16]. They were cut into 5μm thick sections and stained using haematoxylin-eosin dye and finally mounted in di-phenyl xylene. The sections were then examined under microscope for histopathological changes in liver architecture and their photomicrographs were taken.
2.10. Estimation of Oxidative Stress Parameters
2.10.1. Preparation of Liver Homogenate
- The Liver tissues of some of the sacrificed albino rats were excised and the liver samples were cut into small pieces and homogenized in phosphate buffer saline (PBS) to give a 10% (w/v) liver homogenate. The homogenates were then centrifuged at 12,000 rpm for 50 minutes. The supernatant obtained was later used for assay of thiobarbituric acid reactive substances (TBARS) content, superoxide dismutase, catalase and reduced glutathione.
2.10.2. Estimation of Lipid Peroxidative (LPO) Indices
- Lipid peroxidation as evidenced by the formation of TBARS was measured in the homogenate by the method of Niechaus and Sameulsson [17].
2.10.3. Estimation of Superoxide Dismutase (SOD)
- The liver homogenate was assayed for the presence of SOD by utilizing the technique of magwere et al, [18] with slight modification. The assay was performed in 2.5ml of 50m M Na2CO3 buffer to which 0.05ml of the sample was added. 0.3 of the epinephrine stock solution was then added to the above before taking absorbance readings at 480nm for 3-5 minutes. A blank devoid of the samples (but having all the reagents) was used for background correction.
2.10.4. Estimation of Catalase (CAT)
- The liver homogenate was assayed for catalase colorimetrically at 620mm and expressed as µmoles of H2O2 consumed/min/mg protein as described by sinha [19].
2.10.5. Estimation of Reduced Glutathione (GSH)
- Reduced glutathione (GSH) was determined in the liver homogenate using the method of Ellma [20].
3. Data Analysis
- Data analysis was done using the Graph Pad prism computer software. Students‘t’-test and one-way analysis of variance (ANOVA) were used for comparison. A P-value < 0.05 was considered significant.
4. Results
4.1. Analysis of the Gasoline (petrol)
- The table below shows that the petrol used in this research work meet SON/DPR specification.
|
4.2. Preliminary Toxicity Test
- Preliminary toxicity test result shows that one animal died after the administration of 0.5ml of petrol per kg body weight, three died after the administration of 1ml/kg body weight, 5 died after administering 1.5ml/kg body weight and all the eight animals died after administering of 2, 2.5 and 3ml of petrol per kg body weight of the animals respectively. In the control group, none of the animals died since they were given water ad labitum. The calculated LD50 is 0.75ml of petrol per kg body weight of the animals.
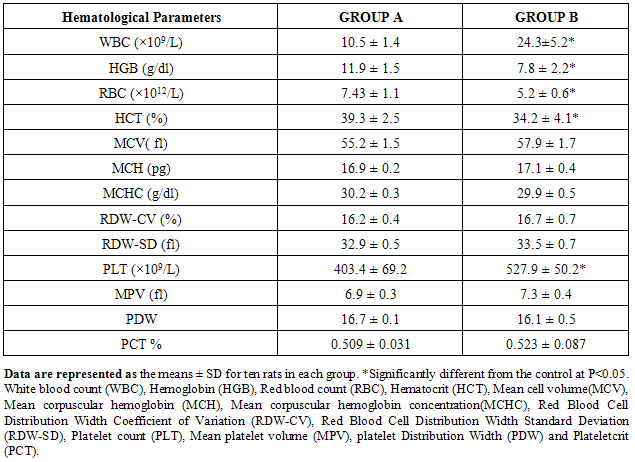
4.3. Effect of Gasoline on Hematological Parameters in Male Albino Rats
- The different hematological parameters of the experimental groups (group A and B) are shown in Table 2 below. The results of the hematological parameters show that the administration of 0.5ml/kg body weight of the animals is hematotoxic to the animals in group B.
|
4.4. Effect of Gasoline on the Liver of Male Albino Rats
- Table 3 below shows the effect of petrol on the liver functions of animals in the studied groups. These liver biomarker enzymes values increased significantly (P<0.05) in group B rats compared to group A rats, these indicate hepatic injury to the liver of animals in group B. There was a significant increase (P<0.05) in the plasma total bilirubin in group B rats and a decrease in plasma total protein level in group B animals compared to group A animals.
|
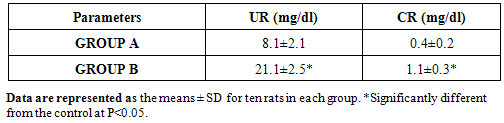
4.5. Effect of Gasoline on the Kidney Functions of Male Albino Rats
- The mean plasma level of urea (UR) and creatinine (CR) significantly increased in group B animals compared to group A animals.
4.6. Histopathological Examination
- Hepatic tissue of rat administered with 0.5ml/kg body weight dose, showing moderate hepatocyte necrosis around the central vein region, liver lesions, fatty changes in rat hepatocytes signifying the existence of hepatosteatosis and mild inflammatory cells. Hepatic tissue of rat administered with 0.5ml/kg body weight of dose, showing moderate hepatocyte necrosis around the central vein region, liver lesions, and fatty changes in rat hepatocytes signifying the existence of hepatosteatosis and severe cell inflammation.
4.7. Effect of Gasoline (Petrol) on Oxidative Stress Parameters
- Figure 4-8 represent the effect of gasoline on oxidative stress indices of animals administered with gasoline. The rats administered with gasoline have high plasma uric acid, high liver homogenate values of malondialdehyde (MDA), low catalase, low superoxide dismutase and low reduced glutathione activities compared to the healthy animals (group A).
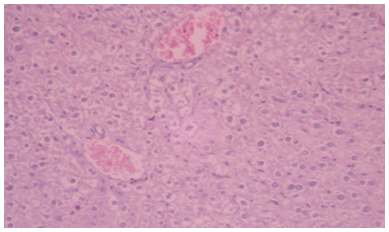 | Figure 1. Photomicrographs of liver sections stained with hematoxylin and eosin. Hepatic tissue of control rat, showing normal appearance and central vein. Haematoxylin and Eosin (H&E) |
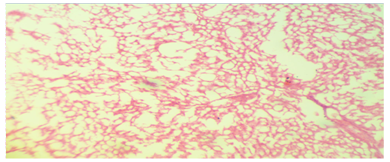 | Figure 2. Photomicrographs of liver sections stained with hematoxylin and eosin. (H&E) |
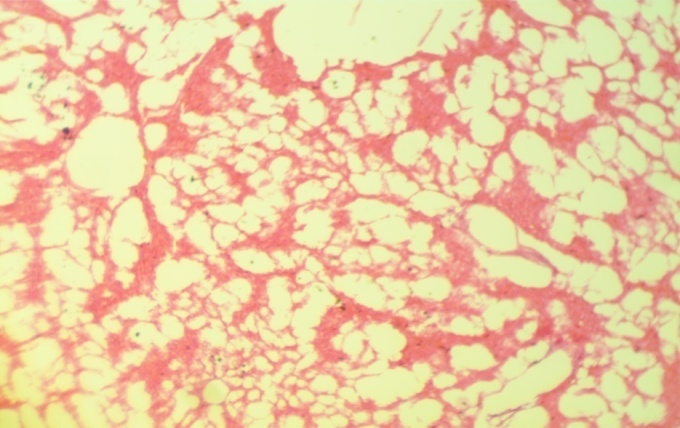 | Figure 3. Photomicrographs of liver sections stained with hematoxylin and eosin. (H&E) |
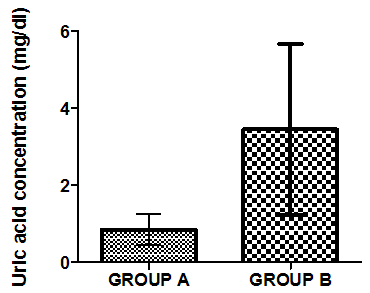 | Figure 4. Mean value of uric acid in liver homogenate of healthy group (group A) and group B animals administered with 0.5ml/kg body weight does of gasoline |
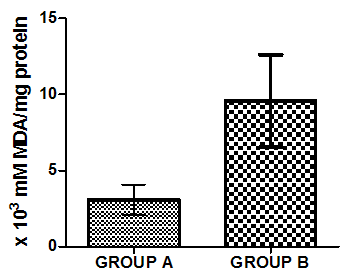 | Figure 5. Mean value of TBARS in liver homogenate of healthy group (group A) and group B animals administered with 0.5ml/kg body weight does of gasoline |
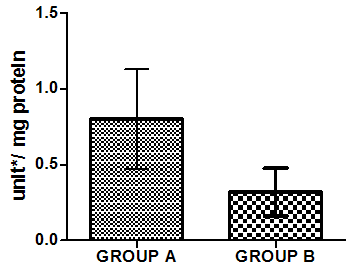 | Figure 6. Activities of Superoxide dismutase in liver homogenate of healthy group (group A) and group B animals administered with 0.5ml/kg body weight does of gasoline |
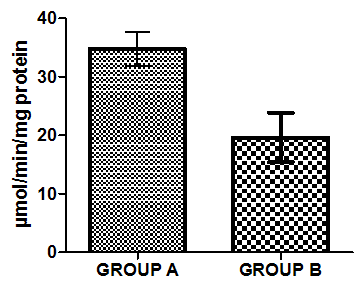 | Figure 7. Activities of Catalase in liver homogenate of healthy group (group A) and group B animals administered with 0.5ml/kg body weight does of gasoline |
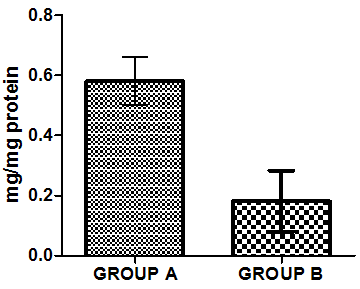 | Figure 8. Mean value of Reduced glutathione in liver homogenate of healthy group (group A) and group B animals administered with 0.5ml/kg body weight does of gasoline |
5. Discussion
- Various environmental pollutants, particularly those associated with crude oil cause many biochemical and toxic effects in terrestrial and marine animals Ngodigha et al. [21].Gasoline is an essential constituent of human life due to their domestic and industrial use.Due to oil spillage, there have been increases in the consumption of these petroleum products. Table I shows that the gasoline used in this study met the SON/DPR Specification. Preliminary toxicity study to determine the volume of petrol that could cause toxicity shows that the animals exhibited changes in behavioural pattern such as salivation, respiratory distress, coma, sedation and death. The LD50 was calculated to be 0.75 ml/Kg body weight dose of gasoline. 0.5 ml of gasoline per kg body weight of the animals was used for the experiment. Ohaeri and eluwa. [22], Sexena et al. [23] and Patrick-Iwuanyanwu et al. [24] reported that hematological and biochemical indices are reliable parameter for assessment of the health status of animals.The primary function of white blood cells appears to be defending the body against foreign bodies, which is achieved by leucocytosis and antibody production [25]. There was a significant increase (P<0.05) in the WBC in the animals administered with gasoline compared to the control group. The observation in this study on WBC is similar to our previous findings where WBC values increased in kerosene administered animals, [26]. The primary reason for assessing the RBC is to check the level of anemia and to evaluate normal erythropoiesis. Leighton et al. [27] characterized haemolytic anemia as the hallmark of crude oil toxicity in animals. HGB level shows the amount of intracellular iron present, while HCT, indicates the volume of RBC in 100ml of blood and it helps to determine the degree of anemia or polycythaemia. The study shows that there is a significant decrease (P<0.05) in RBC, HGB and HCT in the group administered with gasoline compared to the normal control group (Table 2). The significant reduction (P < 0.05) may be attributed to the cytotoxic effects and suppression of the erythropoiesis caused by constituents of the gasoline. The values of the PLT increased significantly (P<0.05) in group B animals compared to group A animals. The overproduction of platelets (thrombocytosis) is usually a transient event and may be related to oxidative stress, inflammation, spleen disorder, reverse effects of thrombopenia, iron deficiency and some types of anemia. Changes in the number of platelets (thrombocytes) reveal disturbances in organism hemostasis and indicate hazardous conditions to which the organisms are exposed to. Evans. [28], reported that excessive increase in platelets could be related to stimulation of megakaryocytes in the bone marrow. Other hematological parameters like MCV, MCH, MCHC, RDW-CV, RDW-SD, MPV, PDW and PCT showed no significant difference (P<0.05) between group A and B animals. It is therefore concluded that gasoline is highly toxic and are potential damaging agents to the hematopoietic system.AST is an enzyme found mostly in the liver cell, heart muscle, skeletal muscles and kidneys. Injury to these tissue result in the release of the enzyme in the blood stream. The result from this study showed significant increases (P<0.05) in the plasma levels of AST and ALT (Table 3) values in group B animals compared to group A animals. This result is in accordance with our previous report [26], where we reported increased in plasma AST and ALT after administering of kerosine on wistar albino rats. This result is in accordance with the reports of Sese et al. [29] who reported similar increase in AST and ALT level after administering Bonny light crude oil to male Chinchilla rabbits. There were significant increase (P<0.05) in the ALP and GGT values of group B rats compared to the healthy rats. This may imply that damage occur in the liver cells of the animals administered with gasoline since the activities of these enzymes are reported to be increased in liver damage. This result is in accordance with our previous report [26], where we reported increased in plasma ALP and GGT after administering of kerosine on wistar albino rats. The significant increase in these liver marker enzymes (AST, ALT, ALP and GGT) in the plasma is responsible for the severe hepatotoxicity of the liver in the animals administered with gasoline. Bilirubin is the major breakdown product that results from the destruction of old red blood cells. It is removed from the blood by the liver; hence it is a good indicator of the function of the liver [30]. The total bilirubin values increased significantly (P<0.05) in group B animals compared to group A animals. Higher bilirubin plasma levels observed in this study may be caused by induction of bilirubin production in response to oxidative stress. But these high plasma levels of bilirubin may not reduce the neurological damage caused by oxidants produced by the gasoline, possibly because the antioxidant activity of bilirubin is in the serum while the actual oxidative damage occurs in the tissue where bilirubin may not penetrate in sufficient concentration to produce anti-inflammatory and antioxidant actions. Nelson and Cox. [31] reported that a rise in plasma level of bilirubin suggests liver cell damage, since liver cells are responsible for removing bilirubin from serum. Ovuru et al. [7] reported an increase in total serum bilirubin concentration in semi adult rabbits exposed to crude oil contaminated diet and attributed this to a metabolic disturbance in the liver arising from defective conjugation and or excretion of bilirubin. The plasma total protein levels increased in the healthy animals (group A) compared to the group administered with gasoline (group B). A general decrease in the total protein value of animals administered with gasoline compared to healthy animals signifies malnutrition, malabsorption, liver disease and kidney disease. The Histopathological findings, evident in the photomicrographs, elucidated the deleterious effect of gasoline on the liver of animals in group B. This result suggests that gasoline has the potential to cause biochemical toxicity and can affect the micro-architecture of the liver. In the healthy animals, liver sections showed normal hepatic cells with well preserved cytoplasm, prominent nucleus, nucleolus and central vein (Figure 1). The administration of 0.5ml of gasoline per kg body weight of the animals induces severe histopathological damages in the liver with significant degeneration of cells, fatty changes in rat hepatocytes signifying the existence of hepatosteatosis, severe cell inflammation, cirrhosis, hepatocyte fibrosis, hepatocyte necrosis and liver lesions were observed in group B animals (Figure 2 and 3).Plasma and urinary concentrations of urea and creatinine are indices of renal function. This study showed significant increased (P<0.05) in plasma urea and creatinine level of group B rats compared to group A rats (Table 4). Ovuru et al. [7] and Ravnskov et al. [32] clinical and experimental studies showed that the exposure of petroleum hydrocarbon is a risk factor for renal diseases. A rise in serum level of these metabolites suggest the inability of the kidney to excrete these products, which further suggest a decrease in glomerular filtration rate (GFR). This affirmation is supported by Counts et al. [33] who reported that chemically induced nephrotocity by halogenated hydrocarbons injure of the proximal tubule monolayer, resulting in gaps in epithelial lining, leading to back leak of filtrate and diminishing glomerular filtration.
|
6. Conclusions
- The results of this research work shows that administration of gasoline has severe effects on hematological parameters, causes impairment of the liver and kidney functions and result to oxidative damage in wistar albino rats.
7. Recommendations
- The authors recommended that gasoline should not be ingested directly or indirectly because of its harmful effect on health.
ACKNOWLEDGMENTS
- The authors are grateful to Miss Chima Lynda Chisorom, a staff from SGS Inspection Services Nigeria Limited for her moral assistance when carrying out this research work. Special greetings to Mr Musa Abdullahi Aiyegbeni, Mr Hassan Danlami Umar and Ademoroti Adenike Ester for their assistance when carrying out this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML