-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(5): 93-97
doi:10.5923/j.ajb.20140405.01
Ameliorating Effect of Moringa oleifera Leaf Extract on Chlorpyrifos-Induced Toxicity in the Brain of Wistar Rats
Oluwole Israel Oyewole, Bolanle Fatimat Olabiyi
Department of Biochemistry, Faculty of Basic and Applied Sciences, Osun State University, Osogbo, Nigeria
Correspondence to: Oluwole Israel Oyewole, Department of Biochemistry, Faculty of Basic and Applied Sciences, Osun State University, Osogbo, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The present study investigated the effects of sub lethal concentration (20 mg/kg bw) of Chlorpyrifos on selected brain parameters (enzymes, protein, histology and antioxidant status) in rats. We also evaluated the possible ameliorating potential of Moringa oleifera leaf extract on Chlorpyrifos-induced oxidative toxicity in rat brain. Eighteen male Wistar albino rats were divided into 3 groups; Group A-control, Group B were administered 20 mg/kg/bw Chlorpyrifos while group C received a combination of 20 mg/kg/bw Chlorpyrifos and 200 mg/kg/bw leaf extract of Moringa oleifera orally for 21 days. Chlorpyrifos caused significant reduction in brain total protein, albumin and globulin while it elevated alkaline phosphatase (ALP) and acid phosphatase (ACP). There was also a significant reduction in reduced glutathione (GSH), glutathione-S-transferase (GST), ascorbic acid, catalase (CAT), superoxide dismutase (SOD) as well as elevated malondialdehyde (MDA) level in the brain of the rats administered the pesticide. In addition, Chlorpyrifos significantly distorted brain histology as characterized by mild development of amyloid plaques and spongy architecture due to vacuole formation in the neurons. Coadministration of Moringa oleifera leaf extract with Chlorpyrifos caused a significant restoration in brain enzymes and protein as well as restoration of antioxidant capacity. Brain architecture was also well preserved in rats who received the drug combination. We conclude that Moringa oleifera leaf significantly reduced chlorpyrifos-induced oxidative stress in rat brain in addition to restoration of protein and enzymes status already distorted by chlorpyrifos.
Keywords: Chlorpyrifos, Moringa oleifera, Antioxidants, Brain histology, Proteins
Cite this paper: Oluwole Israel Oyewole, Bolanle Fatimat Olabiyi, Ameliorating Effect of Moringa oleifera Leaf Extract on Chlorpyrifos-Induced Toxicity in the Brain of Wistar Rats, American Journal of Biochemistry, Vol. 4 No. 5, 2014, pp. 93-97. doi: 10.5923/j.ajb.20140405.01.
Article Outline
1. Introduction
- Epidemiological studies in recent years have shown that exposure of humans to pesticides may increase prevalence of neurological dysfunctions and cancer [1]. It has been estimated that about 3 million cases of pesticide poisoning occur every year resulting in more than 250,000 deaths which may be higher in developing countries where the use of pesticide is particularly widespread due to hot climate condition [2]. Several studies has found significant positive associations between pesticide exposure and brain cancer [3]. Pesticide-induced oxidative stress has been a focus of toxicological research for the last decade as a possible mechanism of toxicity [4]. Several studies have been conducted to determine whether oxidative stress in humans or animals results from various agents in the group and is associated with their toxic effects [5]. Chlorpyrifos (O,O-diethyl O-3,5,6-trichloropyridin-2-yl phosphorothioate) is a crystalline broad-spectrum, chlorinated organophosphate insecticide. It was introduced in 1965 by Dow Chemical Company and widely used indoors and outdoors to control fleas, insects, termites, pests and mosquitoes. Unfortunately, this pesticide can get into the body of man unintentionally when touched or inhaled. Many putative mechanisms have been implicated in molecular mechanisms of chlorpyrifos toxicity out of which the induction of oxidative stress has received tremendous attention [6]. The pesticide acts on the nervous system by inhibiting cholinesterase, the enzyme which catalyze the breakdown of acetylcholine, a neurotransmitter [7]. The resulting accumulation of acetylcholine in the synaptic cleft causes overstimulation of the neuronal cells and interference with normal nerve transmission in the brain [8]. A number of medicinal plants have been reported to possess reactive oxygen species (ROS) scavenging and cytoprotective properties. Moringa oleifera leaves has been shown to posses many pharmacological and physiological activities such as antioxidants, anti-inflammatory, analgesic, anti-carcinogenic and cardiotonic effects which account for its usefulness in traditional medicine for a number of disorders [9]. In the present study, we evaluated the effect of Moringa oleifera in Chlorpyrifos induced oxidative stress and toxicity in the brain of wistar rats.
2. Materials and Methods
2.1. Preparation of Plant Extract
- Fresh Moringa oleifera leaves were obtained from National Centre for Genetic Resources and Biotechnology (NACGRAB), Ibadan Nigeria and air dried at room temperature for 3 weeks. The dried leaves were grinded into powder with a Binatone 1200QX electric blender. 500g of the powdered sample was soaked in 3.0litre of 80% methanol for 3 days and then filtered using a moslin cloth. Crude extract was obtained by evaporating the solvent in a rotatory evaporator at a temperature of 80℃.
2.2. Drug Preparation
- The pesticide used in this experiment is a product of Rocket Agricultural Chemicals, Nigeria. The pesticide contain chlorpyrifos (O,O-diethyl O-3,5,6-trichloropyridin- 2-yl phosphorothioate) as active ingredient (50 g/250ml). The administered dose of 20 mg/kg bw was prepared from the pesticide using distilled water.
2.3. Experimental Design
- Eighteen (18) male albino rats (Rattus novergicus) average weight 140 g were obtained from the Central Animal House, Osun State University, Osogbo Campus. The animals were housed in wooden cages and maintained under normal laboratory conditions (12 hrs light, 12 hrs dark, temperature 24-28℃, relative humidity 60-70%). They were fed with normal rat pellets and clean water ad libitum. The rats were randomly divided into 3 groups of 6 rats each. Group A-control received distilled water, Group B were administered 20 mg/kg/bw Chlorpyrifos while group C received a combination of 20 mg/kg/bw Chlorpyrifos and 200 mg/kg/bw leaf extract of Moringa oleifera orally for 21 days.
2.4. Preparation of Brain Homogenates
- Rats were made unconscious in a jar containing cotton wool soaked with chloroform. The rats were quickly dissected and the brain removed. The excised brain was rinsed in ice cold 1.15% KCL and homogenized in 4 volumes of ice-cold 0.1 M phosphate buffer (pH 7.4). It was then centrifuged at 12,500 rpm for 15minutes at 4℃ in a refrigerated centrifuge to obtain the post mitochondria brain fraction. The homogenates was stored in the refrigerator before use.
2.5. Biochemical Assay
- Total protein, albumin and globulin concentrations in brain homogenate were estimated using standard procedure earlier described [10-12] respectively. Alkaline phosphatase and acid phosphatase were activities measured were measured according to the methods described by [13] and [14] respectively. Ascorbic acid concentration in the brain homogenate was assayed colorimetrically using folin phenol reagent [15]. SOD activity was determined based on the ability of the enzyme to inhibit auto-oxidation of epinephrine at pH 10.2 and 30℃ [16]. Catalase activity was determined by measuring reduction of dichromate in acetic acid to chromic acetate at 570 nm [17]. GST activity was determined using 1,2-dichloro 4-nitrobenzene (CDNB) as substrate [18]. GSH level in the brain sample was estimated as earlier described [19]. Thiobarbituric acid reactive substances (TBARS) was measured as an indicator of lipid peroxidation and expressed as micromolar of malondialdehyde (MDA)/g tissue [20].
2.6. Histological Examination
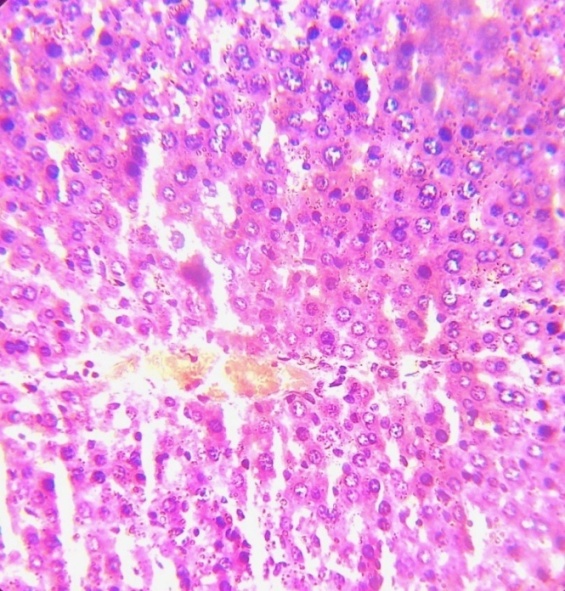
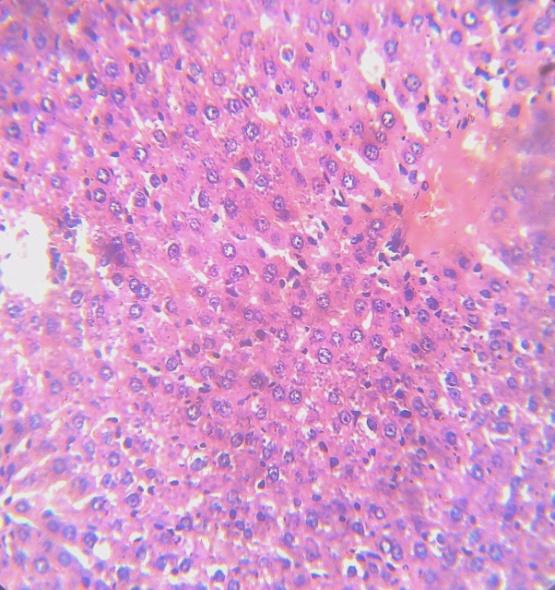
- The method of Bancroft and Stevens [21] was employed for processing of brain tissues for histological examination. A fine section of the brain was cut with a clean, sterile scissors and immediately transferred into 10% (v/v) formaldehyde saline solution. 7-10 mm semi-serial cuts slice tissues was dehydrated through ascending grades of ethanol (70%, 90% and 95% v/v), cleaned in xylene, and embedded in paraffin wax. It was stained with haematoxylin and eosin and mounted in a Leitz microscope and their photomicrograph taken at x 200 with a Canon (Meville, NY) Power Shot G2 digital camera.
3. Results
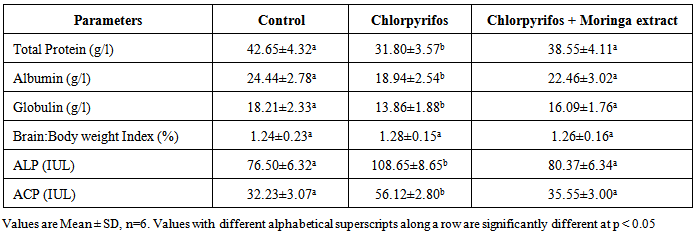
- The results of proteins and enzyme concentrations in the brain homogenate of control and test groups animals is shown in Table 1. Chlorpyrifos significantly reduced the concentrations of total protein, albumin and globulin in the rats brain accompanied with elevation of ALP and ACP enzymes. These alterations were significantly normalized in rats treated with Moringa oleifera close to that obtained in the control. There was no significant difference in the brain: body weight index of rats administered the pesticide compared to the control indicating that the pesticide did not cause brain enlargement or reduction.
|
|
4. Discussion
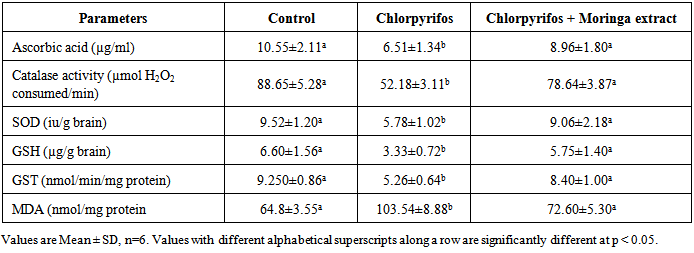
- Chlorpyrifos caused cellular dysfunction in the brain of rats in this study as it significantly reduced brain antioxidant capacity, elevated lipid peroxides and caused distortion of brain histology. This results indicate its ability to induce oxidative stress in the brain. The pesticide also depleted protein content in rats brain as it significantly reduced total proteins, albumin and globulin. These results implicate the pesticide as a potent brain toxicant. Pesticides are believed to damage the lipoidal matrix in cell, generating reactive oxygen species (ROS) and promoting oxidative stress. Excess production of ROS can cause oxidative modification of macromolecules (proteins, DNA, and lipids) and decreased level of endogenous antioxidants leading to accelerated cell death [22]. Chronic exposure to chlorpyrifos has been linked to neurological effects, developmental disorders, and autoimmune disorders [23] Exposure during pregnancy has been reported to affect mental development of children [24]. It has been observed that the relative rate of detoxification of chlorpyrifos is lower in rats and mice compared to other mammals which may account for the increased toxicity of chlorpyrifos in these animals [25]. The decrease in total protein, albumin and globulin observed in rats administered chlorpyrifos (group B) with no proportional decrease in brain weight might be due to its toxicity on the liver which affects protein production as suggested by previous workers [26]. The observed significant increase in the activities of ALP and ACP in the brain of rats administered chlorpyrifos alone suggests that the pesticide had stimulatory action on the enzymes in these tissues. This may arise probably by an indirect effect of stabilizing the membrane, which makes their localized environment in the membrane more conducive to enhance their action. The increase might also be due to the response of the brain system to offset the stress introduced by exposure to the drugs. It has been reported that increased activity of various enzymes was observed under conditions of stress in animal tissues [27].Antioxidant levels, proteins and enzymes in the brain of rats treated with Moringa oleifera shows a normal range not significantly different from that obtain in the control rats (group A). Normal brain histology was also restored in rats treated with the extract. This shows the effectiveness of Moringa oleifera in the treatment of oxidative stress [28]. The mammalian cells are equipped with mechanisms to reduce the adverse effect of lipid peroxidation by making use of both enzymatic and non-enzymatic antioxidants, which scavenge for free radicals [29]. Oxidative stress results when the endogenous antioxidants have been overwhelmed by the amount of free radical generation. Therefore the increase in exogenous supply of antioxidants by Moringa oleifera in this study is an indication of improvement of brain capacity to cope with free radicals.
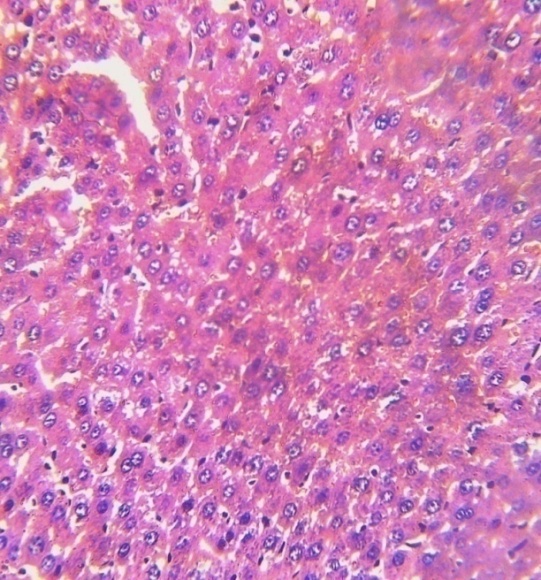 | Plate 1a. Section of brain cerebrum of normal control rats (x200) stained with haematoxylin and eosin. Picture reveal normal colour and architectural structure of the cerebrum |
5. Conclusions
- This study provide an evidence of oxidative damage and significant impairment of normal brain function in rats by chlorpyrifos. It suggests an intriguing possibility that repeated exposure to sub lethal doses of chlorpyrifos increases brain dysfunction through oxidative stress. Administration of Moringa oleifera however has protective effects on chlorpyrifos-induced brain toxicity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML