-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(4): 84-92
doi:10.5923/j.ajb.20140404.04
Characterization of a Novel α-amylase from Bacillus macquariensis Isolated from Cassava Peels Dump Site
Adekunle Toyin Bamigbade1, Isaac Olusanjo Adewale1, Mufutau Kolawole Bakare2
1Department of Biochemistry, Obafemi Awolowo University, Ile-Ife, Nigeria
2Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence to: Isaac Olusanjo Adewale, Department of Biochemistry, Obafemi Awolowo University, Ile-Ife, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In search for hydrolytic enzymes with novel catalytic ability from agricultural waste materials, a moderately thermostable α-amylase from Bacillus macquariensis isolated from cassava peels dumpsite has been purified and characterized. The enzyme was purified in two steps using ion exchange and gel filtration chromatography. The purified enzyme appeared to be a dimer with a native molecular weight estimate of 63.4 ± 1.27 kDa on gel filtration while the subunit molecular weight gave 28.9 kDa. The apparent Km and Vmax of this enzyme for soluble starch were estimated, to be 106.8 ± 10.08 mg/ml and 4380 ± 222 units/mg protein respectively. The enzyme was not readily saturated with gelatinized starch at concentrations as high as 14%. The optima temperature and pH established for amylolytic activity in this study were 55°C and 6.0 respectively while the activation energy for starch hydrolysis (Ea) was 41.5 ± 0.5 kJK-1. The enzyme retained more than 70% of its maximum activity over a broad range of pH 4.5–9.0 at room temperature for 60 min. This study revealed the purified α-amylase to be an enzyme in which activity and heat stability were not stimulated by an inclusion of exogenous Ca2+.
Keywords: Hydrolytic enzyme,Agricultural waste, Bacteria, α-amylase, Cassava peels
Cite this paper: Adekunle Toyin Bamigbade, Isaac Olusanjo Adewale, Mufutau Kolawole Bakare, Characterization of a Novel α-amylase from Bacillus macquariensis Isolated from Cassava Peels Dump Site, American Journal of Biochemistry, Vol. 4 No. 4, 2014, pp. 84-92. doi: 10.5923/j.ajb.20140404.04.
Article Outline
1. Introduction
- Alpha amylases (EC 3.2.1.1) are one of the most important industrial enzymes that have diverse applications in a wide variety of industries such as food, fermentation, textile, paper, detergent, pharmaceutical and sugar industries [1, 2]. This enzyme is present in animals, plants, bacteria and fungi [3] although amylases of microbial origin are better suited for industrial uses [4]. Commercial production of amylases from microorganisms represents 25–33% of the world enzyme market [5, 6]. The two genera commonly employed for industrial purposes are Bacillus and Aspergillus; these include B. licheniformis, B. amyloliquefaciens, B. stearothemophilus, B. subtilis, and A. niger [7, 8]. Generally, fungal α-amylases are mainly saccharifying enzymes while bacterial α-amylases are liquefying enzymes. Saccharifying enzymes produce a higher degree of depolymerization [9]. Alpha Amylase degradation of starch gives rise to α-limit dextrins, glucose and small oligosaccharides [10]. Microorganisms capable of producing amylases with efficient starch hydrolytic properties can be isolated through an objective approach from agro-wastes such as bran and husk of grains and cassava peels. Amongst the desirable properties sought for in industries are low pH stability, higher thermostability, Ca2+ independence of both enzyme stability and activity, and utilization of high concentration of starch. One or a combination of which can be very useful in related applications [2, 11, 12]. In the course of conventional enzymatic saccharification, a slurry containing 15% starch is gelatinized leading to an increase in slurry viscosity thereby posing a challenge with mixing and pumping [13, 14]. This engenders a need for α-amylase with atypical kinetics able to hydrolyse starch effectively at this concentration. Thus far, majority of the reported α-amylases are readily saturated at low starch concentration and further increment tend to inhibit activity; hence may not be suitable for industries with bulk starches. We report here some molecular properties of an atypical α-amylase from Bacillus macquariensis obtained from cassava peels dumpsite suggestive of its potentials in bulk starch and detergent industries.
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
- Bovine serum albumin (BSA), D-glucose, Ovalbumin, Peroxidase, Lysozyme, α-Chymotrypsinogen A, Potassium sodium tartarate and Coomasie brilliant blue R250 were obtained from Sigma Chemical Company, Limited, St. Louis, MO, USA. Blue Dextran, CM Trisacryl were obtained from Pharmacia, Fine Chemicals AB, Uppsala, Sweden. Bio-Gel P-60 was from Bio-rad Laboratories, Richmond, California. Sodium dodecyl sulphate (SDS) was obtained from Pierce Chemical Company, Rockford, Illinois, USA. Acrylamide, N,N-methylene bisacrylamide (MBA) and Ammonium persulphate were from Serva Feinbiochemica, Germany. N,N,N1,N1-tetramethylethylenediamine (TEMED) was from Eastman Kodak, Rochester, N. Y., USA.
2.1.2. Methods
- Production of Bacillus macquariensis α-amylase The enzyme production was carried out in 250 ml Erlenmeyer flasks containing 100 ml medium using 1ml of standardized inoculum. The medium consisted of 1 g soluble starch, 0.1 g KH2PO4, 0.25g Na2HPO4, 0.1g NaCl, 0.005 g MgSO4·7H2O, 0.005 g CaCl2, 0.2g (NH4)2SO4 and 0.2g peptone. The initial pH of the medium was adjusted to 6.5 and the medium was sterilized at 121°C for 15 minutes. The isolate was grown at 45°C for 48 h in 100 ml production medium and agitated at 150 rpm. Cell-free supernatant (CFS) was obtained by centrifugation at 6000 x g for 30 min. The CFS was used as enzyme source.
2.1.3. Alpha Amylase Assay
- The α-amylase activity assay was performed at 25°C in 10 mM phosphate buffer pH 6.5 for 20 min. Briefly, one ml of reaction mixture consisting of 0.1 ml of 1% (w/v) soluble starch in 0.86 ml of 10 mM phosphate buffer (pH 6.5), and 0.04 ml of enzyme solution was incubated for 20 min at 25°C. The α-amylase activity was routinely estimated by the measurement of the reducing sugar liberated according to the arseno-molybdate method of Somogyi [15] at 540 nm. One unit of α-amylase is defined as the amount of enzyme releasing 1.0 μg glucose equivalence from the substrate per minute at room temperature under the standard assay condition.
2.1.4. Protein Assay
- Protein concentration was determined by Bradford method [16] with bovine serum albumin as the standard protein.
2.1.5. Enzyme Purification
- Eighty nine milliliter (89 ml) of cell free supernatant obtained from a batch culture was concentrated by lyophilization and re-dissolved in 10 mM phosphate buffer pH 6.5. The re-dissolved enzyme solution was layered on CM Trisacryl column (1 cm x 10 cm) which had previously been equilibrated with 10 mM phosphate buffer, pH 6.5. Fractions were collected at a flow rate of 10 ml/hr and bound proteins were eluted with a linear gradient of 0 to 1.0 M NaCl. Alpha Amylase activity in the fractions and the protein profile were determined. Active fractions in a peak were pooled, and lyophilized.Size exclusion chromatography was performed with Bio-Gel P-100 in a 1 cm x 48 cm column. The column was washed with equilibrating buffer (20 mM tris buffer pH 7.0) and the re-dissolved concentrated enzyme from the previous purification step was subsequently applied. The elution was carried out using the same buffer at a flow rate of 10 ml/hr. Fractions with α-amylase activity were similarly pooled and used for the characterization.
2.1.6. Native and Subunit Molecular Weight Determination
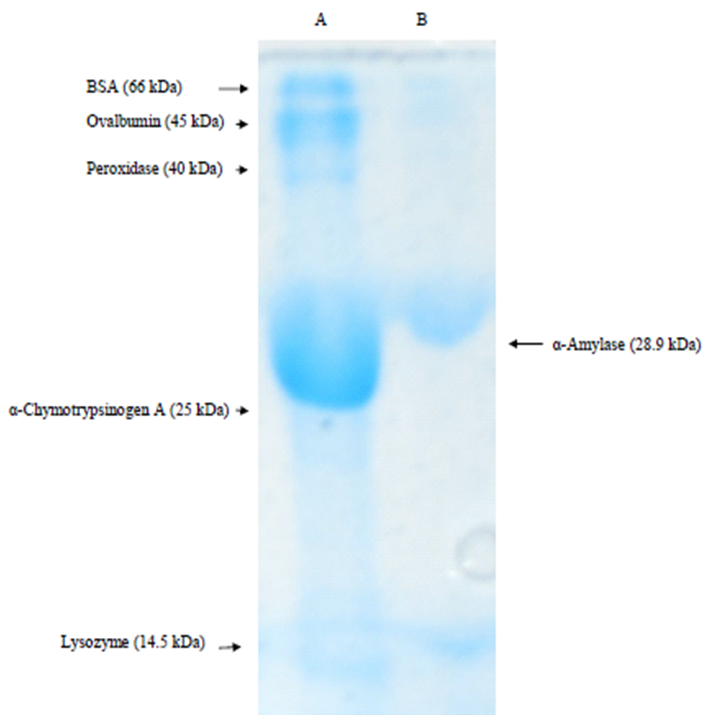
- The subunit molecular weight of the purified α-amylase was determined using SDS-PAGE method of Laemmli [17]. The native molecular weight was determined on a Bio-Gel P-100 gel filtration column. The following protein standards were used: lysozyme (14.5 kDa), α-chymotrypsinogen A (25.6 kDa), peroxidase (40 kDa), ovine albumin (45 kDa), and bovine serum albumin (67 kDa).
2.1.7. Determination of Kinetic Parameters
- The apparent kinetic parameters (Km and Vmax) were determined for the purified Bacillus macquariensis α-amylase by incubating an aliquot of the enzyme with varied concentrations of soluble starch (0 - 140 mg/ml starch) while keeping all other components constant. The data obtained were analyzed using Graph pad prism 5.
2.1.8. Effect of Temperature on Activity of B. macquariensis α-amylase
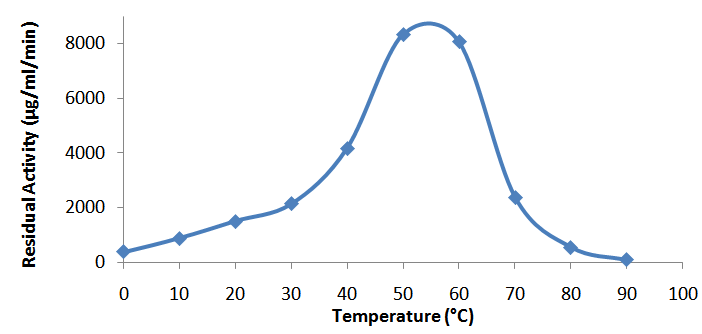
- The effect of temperature on α-amylase activity was studied by incubating an aliquot of the enzyme with the substrate at temperatures ranging from 0 to 90°C for 20 min. The residual α-amylase activity was plotted against the different temperatures.
2.1.9. Stability of Bacillus macquariensis α-amylase Against Heat Inactivation
- Thermal stability of the purified enzyme was also examined by incubating the enzyme preparation for 60 min at different temperatures (50 to 65°C). Aliquots were withdrawn at 10 min intervals and the residual activity was measured under standard assay conditions earlier stated. The residual activities were expressed as a percentage of the activity at zero time which was taken to be 100%. The percentage residual activity was plotted against time of incubation.
2.1.10. Effect of pH on activity and Stability of Bacillus macquariensis α-amylase
- The effect of pH on α-amylase activity was investigated within the pH range of 4.0 – 9.0 at room temperature. For the measurement of pH stability, the enzyme was incubated at room temperature for 1 h in buffers at different pH values and the residual activity was determined. The following buffer systems at the indicated pH values were used: 10 mM acetate buffer, pH 4.0–5.5; 10 mM phosphate buffer, pH 6.0–7.5 and 10 mM tris buffer, pH 8.0 – 9.0.
2.1.11. Effect of Metal Ions on B. macquariensis α-amylase
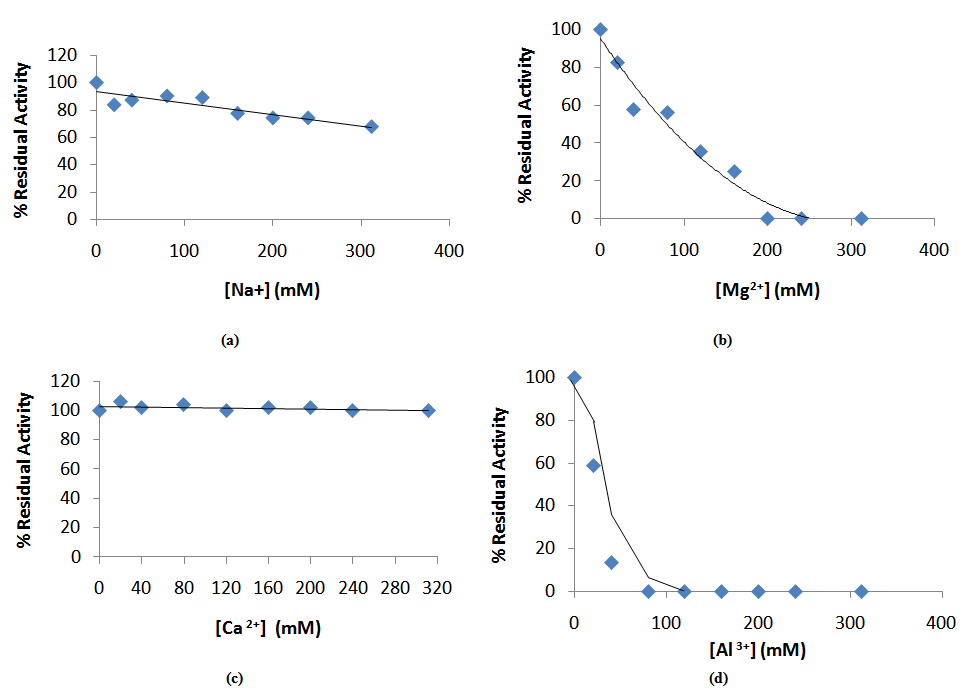
- The effect of mono, divalent, and trivalent metal ions on Bacillus macquariensis α-amylase activity was determined by incubating the enzyme in 10 mM phosphate buffer, pH 6.5 containing the metal ions: NaCl, MnCl2, MgCl2 CaCl2, and AlCl3 together with the appropriate control without metal ion. The enzyme activity was expressed as a percentage of the control which was taken to be 100%.
2.1.12. Effect of EDTA on the α-amylase Activity
- The effect of ethylenediaminetetra-acetic acid (EDTA) on the activity of α-amylase was determined by assaying the enzyme in the presence of EDTA at 0 – 320 mM concentrations. The residual activity was also expressed as a percentage of the control which was taken as 100%.
3. Results
3.1. Purification of the α-amylase
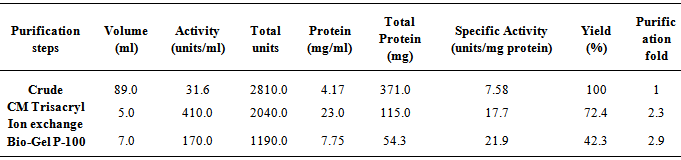
- In the preliminary experiments conducted to choose the best method for the concentration of the cell-free crude supernatant, lyophilization gave the highest recovery and was subsequently adopted for other steps requiring concentration.Purification of the concentrated enzyme on CM Trisacryl cation exchanger gave a single activity peak (Fig. 1a) with a yield of 72.4%. The final purification step on Bio-Gel P-100 size exclusion column gave a yield of 42.3%. The band that corresponded to the purified amylase showed a subunit molecular weight of 28.9 kDa (Fig. 2). The native enzyme has a molecular weight of 63.4 ± 1.27 kDa. Together the results suggest that the amylase is a dimeric protein. The results of the purification procedure are summarized in table 1.
|
3.2. Kinetic Parameters of Purified Amylase
- Apparent Km and Vmax for the α-amylase were estimated using Graph-pad prism 5 to be 106.8 ± 10.08 mg/ml and 4380 ± 222 units/mg protein respectively. The activity of the purified α-amylase increased with increase in substrate concentration until a maximum was reached at 130 mg/ml of soluble starch. Above this concentration, the enzyme appears to be saturated.
3.3. Effect of Temperature on the Enzyme Activity and Stability
- The optimum temperature for the enzyme activity was 55°C. The activity increases gradually from 0°C to the optimum temperature. Beyond this optimum temperature, the activity declined gradually and at 90°C, the activity had come down to 0 (Fig. 3a). The activation energy, Ea, for the purified α-amylase was estimated at 41.5 ± 0.5kJ K-1. 55% of the original enzyme activity was retained after 40 minutes of incubation at 50°C. However, about 70% of the enzyme activity was lost upon 10 min incubation at 65°C and the enzyme was totally inactivated at 50 min (Fig. 4).
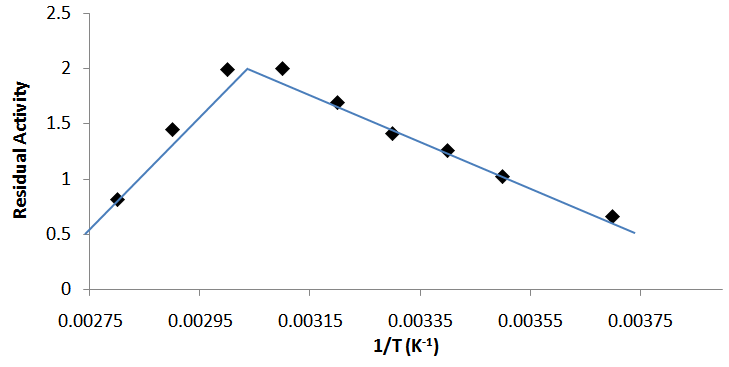 | Figure 3b. Arrhenius plot for the determination of the activation energy (Ea) of purified Bacillus macquariensis α-amylase |
3.4. Effect of pH on the Enzyme Activity and Stability
- The enzyme was active at pH of 6.0 and more than 50% of the activity was retained in the pH range of 4.5 to 8.0 (Fig. 5). The enzyme was relatively stable over the pH range examined retaining more than 70% of optimum stability between pH 4.5 – 9.0. Optimal pH of the enzyme was observed at 5.5.
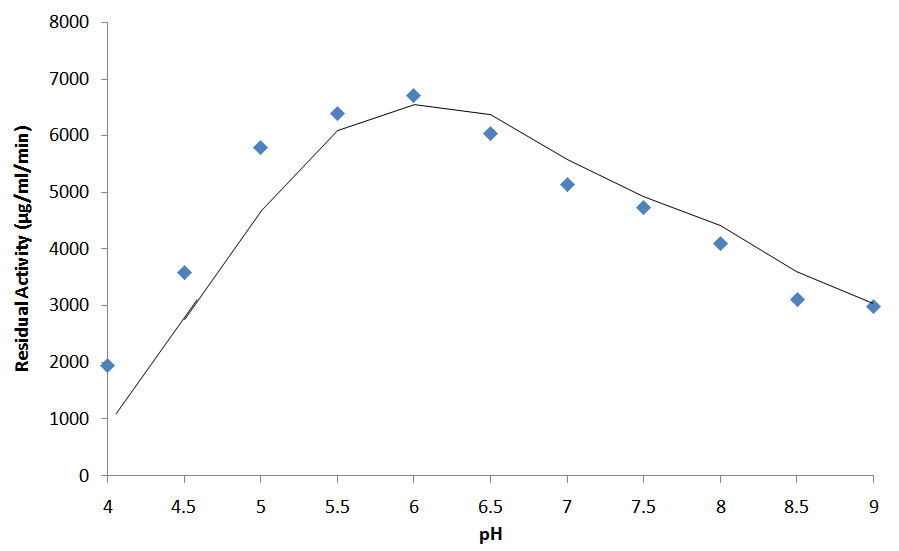 | Figure 5. Effect of pH on the activity of purified Bacillus macquariensis α-amylase. The enzyme was assayed for activity at the indicated pH values at 25°C |
3.5. Effect of Metal ions and EDTA on Bacillus macquariensis α-amylase Activity
- The effect of metal ions on the activity of purified Bacillus macquariensis α-amylase was investigated by assaying for the activity in the presence of various concentrations of the metal ions in the assay mixture. Mn2+ inhibited the enzyme at all the concentrations investigated. Na+, Mg2+, and Al3+ inhibited the enzyme activity to varying degrees: 67% residual activity was recorded at Na+ concentration of 312 mM (Fig. 6a), while Mg2+ ion at 200 mM (Fig. 6b) and Al3+ ion at 80 mM (Fig. 6c) completely inhibited the enzyme activity. Ca2+ ion had no effect on the α-amylase activity up to 312 mM (Fig. 6d). EDTA inhibited the enzyme activity by over 55% at 40 mM while the level of inhibition remained relatively constant up to 240 mM. At 312 mM more than 80% enzyme activity had been lost.
4. Discussion
- In this study, the crude enzyme obtained from cell free supernatant of B. macquariensis gave a specific activity of 7.58 units/mg protein and final purification step yielded 42.3% of overall recovery with a specific activity of 21.9 units/ mg protein which amounted to a 3-fold purification.The apparent molecular mass of the purified B. macquariensis α-amylase was 63.4 ± 1.27 kDa as estimated by gel permeation chromatography on Bio-Gel P-100. However, when the subunit molecular mass was estimated on SDS-PAGE, the purified enzyme revealed a protein band with an approximate molecular mass of 28.9 kDa. Thus, the B. macquariensis α-amylase appeared to be a dimeric protein. The kinetic parameters reported for bacterial α-amylases vary. This might be due to differences in the source of starch, method of preparation, and assay conditions [18]. The apparent Km and Vmax of B. macquariensis α-amylase for soluble starch were estimated to be as 106.8 ± 10.08 mg/ml and 4380 ± 222 units/mg protein respectively in this work. The high Km value with respect to what is generally reported for Bacillus sp. α-amylases implies that the enzyme would not be easily saturated with substrate. These molecular characteristics are pointers to the potentials of this enzyme in starch processing industry where mashes containing about 15% starch are generally employed [14]. It is worth noting that most of the bacterial α-amylases with similar bulk starch digestibility work best with raw starches [14, 19].The α-amylase of Bacillus macquariensis displayed a broad pH activity profile over a range of 4.5– 8.0 with an optimum at pH 6.0. The relative activities at pH 4.0 and 9.0 were about 28.9 and 44.5% respectively, of that measured at pH 6.0. Alpha amylase of B. licheniformis IFO12196 which displayed optimal activity at pH 6.0, retained about 20% of its maximum activity at pH 9.0 and completely lost its activity at pH 10.0 [20]. Most of the widely studied bacterial α-amylases show maximum activity in the pH range of 5.0 - 7.0 [21, 22, 23]. Though, there are reports on α-amylases with optimum activity at pH 3.0 [24] and pH 10.0 [25].Stability studies revealed that the enzyme retained more than 70% of its maximum activity over a broad range of pH 4.5–9.0 at room temperature for 60 min. The enzyme was optimally active at 55°C with the activation energy for starch hydrolysis (Ea) of 41.5 ± 0.5 kJK-1. This data favor enzyme application in processes that require complete inactivation, such as the baking industry [26]. Activity and stability of α-amylase are known to increase in the presence of CaCl2 probably due to stabilization of the enzyme structure resulting from salting out of hydrophobic residues by Ca2+ in the protein [27, 28]. The stability of purified α-amylase from B. macquariensis, was not improved at 65°C or below in the presence of Ca2+ ion. Also Ca2+ ion have no effect on B. macquariensis α-amylase activity at all concentrations examined. This implies that enzyme activity is Ca2+ independent. Although the existence of a tightly-bound Ca2+cannot be ruled out. Na+, Mg2+, and Mn2+ ions inhibited the enzyme to varying extent. Among the possibilities, the inhibitory effects attributed to Mg2+ and Mn2+ ions might be due to the competition for calcium binding sites while Na+ ion might be a poor competitor for calcium binding [29, 30]. The partial inhibition of the α-amylase observed with EDTA at all concentrations examined probably confirms its metalloenzymic property and the possibility that the metal ion is indeed tightly bound to the enzyme molecule. In conclusion, the purified α-amylase obtained in this study could be employed industrially in bulk starch hydrolysis such as fruit juice clarification, starch desizing, and paper industries because of its atypically high Km value. Also the stability displayed over alkaline pH is an index of its potential in detergent industries, although further work needs to be done to investigate its stability in the presence of detergents. The kinetics of this enzyme might be due to the adaptation of the isolate to its environment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML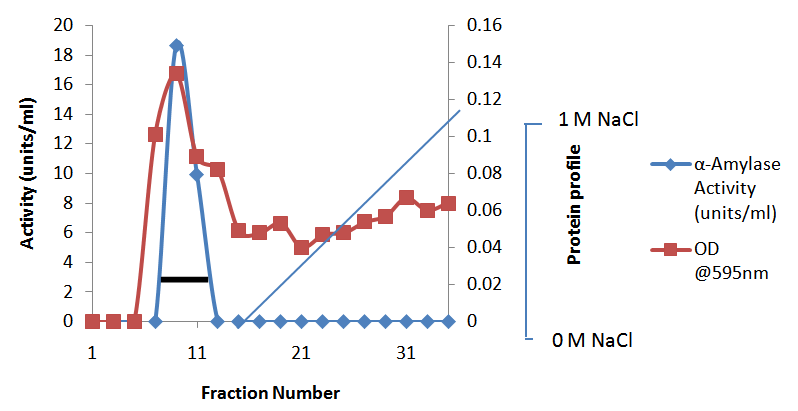
 were determined as described in the text. The profile is indicated by
were determined as described in the text. The profile is indicated by 
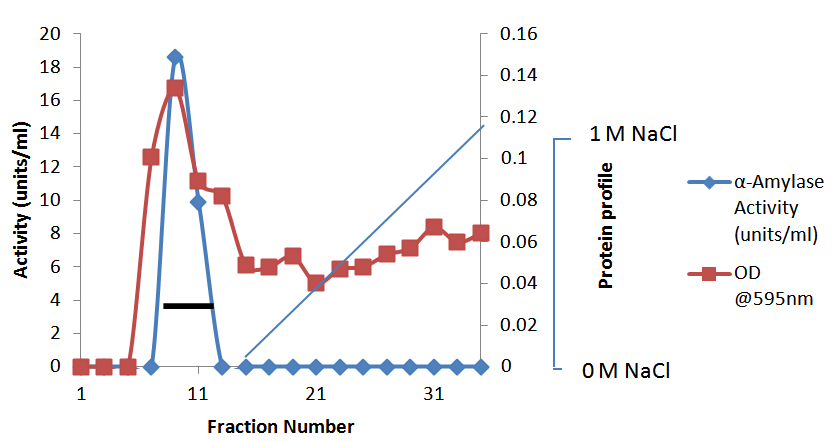
 were eluted at a flow rate of 10 ml/h and fractions of 0.5 ml were collected. Activity is indicated by
were eluted at a flow rate of 10 ml/h and fractions of 0.5 ml were collected. Activity is indicated by 
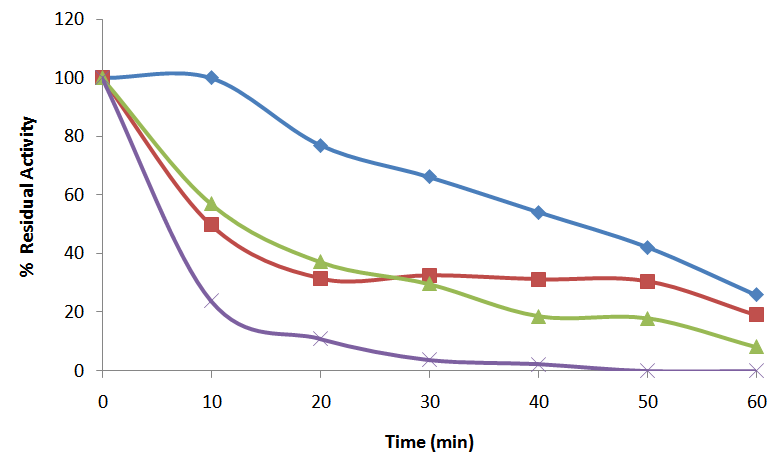
 55°C
55°C  60°C
60°C  , and 65°C (X). At the indicated time intervals, 10 μl of the aliquot was assayed for residual activity determination. The activity obtained was expressed as a percentage of the unincubated control which was taken as 100%
, and 65°C (X). At the indicated time intervals, 10 μl of the aliquot was assayed for residual activity determination. The activity obtained was expressed as a percentage of the unincubated control which was taken as 100%