-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(4): 76-83
doi:10.5923/j.ajb.20140404.03
Biochemical Effects of Hydroclathrus clathratus on Alloxan Induced Diabetic Rats
1Chemistry department, Faculty of science, Beni-Suef University, Egypt
2Biohemistry department, Faculty of Medicine, Azher university, Assuit, Egypt
Correspondence to: Nagy MA, Chemistry department, Faculty of science, Beni-Suef University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Alloxan administration in male albino rats, induced diabetes by increasing blood glucose concentration and reducing hepatic glycogen content as compared to normal control group. Besides, serum lipid profile parameters such as total-cholesterol, triglyceride, low-density lipoprotein and very low-density lipoproteincholesterol were also elevated, whereas, the level of high-density lipoprotein-cholesterol was reduced significantly (P<0.05) in diabetic rats. Treatment of diabetic animals with hot aqueous extract of Hydroclathrusclathratus for 30 days, significantly lowered blood glucose level, elevated hepatic glycogen content and maintained body weight and lipid-profile parameters near normal range.Declined activity of antioxidant enzymes and concentration of non-enzymatic antioxidants were also normalized by (HCE)extract ofHydroclathrusclathratus treatment, thereby reducing the oxidative damage in the tissues of diabetic animals and hence indicating the antidiabetic and antioxidant efficacy of the extract. Biochemical observations were further substantiated with histological examination of pancreas and liver.
Keywords: Hydroclathrus clathratus, Diabetes mellitus, Oxidative stress
Cite this paper: Nagy MA, Mohamed SA, Biochemical Effects of Hydroclathrus clathratus on Alloxan Induced Diabetic Rats, American Journal of Biochemistry, Vol. 4 No. 4, 2014, pp. 76-83. doi: 10.5923/j.ajb.20140404.03.
Article Outline
1. Introduction
- The increasing incidence of diabetes represents an enormous socio-economic burden in the developing countries. The World Heath Organization estimates that over 300 million people worldwide will have Diabetes mellitus (DM) by the year 2025 with alarming proportions from developing countries [1].DM is a chronic disease caused by inherited or acquired deficiency in insulin secretion and by decreased responsiveness of the organs to secrete insulin. There are two main forms of diabetes. Type 1 diabetes is due primarily to autoimmune-mediated destruction of pancreatic islet β-cells, resulting in dramatic insulin deficiency [2]. Oxidative stress represents a significant mechanism for the destruction of cells that can involve apoptosis in a variety of cell types such as endothelial cells (ECs), cardiomyocytes, and smooth muscle cells through multiple cellular pathways. The production of Reactive oxygen species (ROS) can lead to cell injury through a number of processes that involve the peroxidation of cellular membrane lipids [3].Oxidative stress one of the most factors that can ultimately lead to the onset and subsequent complications of DM. Diabetics and experimental animal models exhibit high oxidative stress due to chronic hyperglycemia, which thereby depletes the activity of antioxidative defense system and thus promotes de novo free radicals generation [4]. The source of oxidative stress is a cascade of ROS leaking from the mitochondria. This process has been associated with the onset of type 1 diabetes mellitus (T1DM) via the apoptosis of pancreatic β-cells, and the onset of Type 2 diabetes (T2D) via insulin resistance [5].In recent years due to the adverse effects of synthetic hypoglycemic drugs, interests in alternate therapeutic approaches have become very popular. Nowadays, herbal drugs are gaining popularity in the treatment of diabetes and its complications due to their efficacy, low incidence of side effects and low cost [6].Brown marine algae have been found to be rich sources of anti-oxidant compounds with potential free radical scavenging for the prevention and treatment of various diseases caused by oxidative damage. Fucoidans, polysaccharides containing substantial percentages of L-fucose and sulfate ester groups, are constituents of brown algae that have numerous other biological properties such as anti-inflammatory immunomodulatory and apoptosis-inducing activities [7].Hydroclathrus clathratus (C. Agardh) Howe is a brown marine algae that is considered to be a traditional drug and health food in Korea, Japan and China. Hot water extract of H. clathratus (HCE) are rich in water-soluble sulfated polysaccharides that exhibit several therapeutic effects [8]. In the present study, the effect of 30 day chronic oral treatment with HCE (dose of 400 mg/kg body wt) on diabetes and resultant oxidative stress was investigated by evaluating their antihyperglycemic, antihyperlipidemic and anti-oxidative properties in alloxan induced diabetic rats. This was confirmed through histological examination of the pancreas and liver of alloxan-induced diabetic rats.
2. Materials and Methods
- Chemicals: Alloxan monohydrate was purchased from sigma Fine chemicals (USA). Other chemicals, used for this study were of analytical grade and obtained from Stanbio Laboratory USA Kits. Kits for the estimation of total cholesterol, triglyceride and HDL High density lipoprotein -cholesterol were purchased from Diamond Diagnostic (Egypt).
2.1. Preparation of Extract
- Hydroclathrus clathratus was collected from El Zafrana, Gulf of Suez, Egypt. About 100 g of the fresh alga, corres ponding to 10 – 12 g of dry alga material was homogenized in 500 ml hot double distilled water. The mixture was clarified by filtration using Whatman No.1 filter paper and the light brown extract was obtained. The water extract of H. clathratus were sterilized by filtration and autoclaving, respectively.
2.2. Animal Care and Monitoring
- Healthy male alborats (5-7 months old, weighing 190-210 g) were procured from Faculty of Agricultural, El Minia University, Egypt. They were housed under standard laboratory conditions of light (L:D cycle), temperature (23 ± 2°C) and relative humidity (55 ± 5%). The animals were provided with standard rat pellet feed and tap water ad libitum. Maintenance and treatment of all the animals were performed according to the principles of University’s Animal Ethics Committee that formed as a result of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Egypt.
2.3. Induction of Diabetes and Treatment with Drugs
- For the present study, 30 rats were divided into following 3groups each one contain 10 rats: These groups were the NC (normal control), DC (diabetic control) and HCE (diabetic + H. clathratus extract treated) groups. After the rats were fasted for 18 hours, the rats of group DC and HCE were made diabetic by a single intra-peritoneal injection of alloxan monohydrate (150 mg/kg body wt) that was dissolved in normal saline [9]. Subsequent to alloxan administration, the rats had free access to food and water and were provided with 50% glucose solution to drink overnight to counter HCE- induced hypoglycemic shock. One week after alloxan injection, the fasting blood glucose (FBG) concentration was determined by means of one touch ultra glucometer (Johnson & Johnson Company, USA) and compatible blood glucose strips [10]. Rats showing a FBG level greater than 140 mg/dl were considered diabetic and they were selected for treatment with hot water extracts of Hydroclathrus clathratus (HCE) (400 mg/kg body wt.). HCE was administered (once in a day for 30 days) orally.
2.4. Biochemical Estimations in Blood and Serum Insulin
- Fasting blood glucose (FBG) concentration of all four experimental groups was determined by using a glucometer during different phases of the experiment by withdrawing blood from the tail vein of injected rats. Serum insulin was assayed in the Radioactive Isotopes Unit at the Central Department of scientific Analysis and Test, National Research Center (Dokki, Giza) by radio-immunoassay kits (Diagnostic Products Corporation, Los Angeles, USA) [coat-A-count] [11].For estimating serum lipid profile, serum was isolated from the blood that was collected by cardiac puncture under mild ether anesthesia from overnight fasted rats on 30th day of HCE treatment.
2.5. Biochemical Estimation in Tissue Homogenates
- The rat liver and pancreas were removed, freed from adhering tissues and washed with ice-cold normal saline solution (0.9%) (w/v). Weight of all organs are taken (only) after drying the tissue. 1 g tissue was homogenized in 10 ml of 0.2 M tris-HCl using a homogenizer. The homogenate was filtered and then centrifuged at 10,000 rpm for 20 minutes at 4°C. The supernatant obtained was used for estimation of superoxide dismutase (SOD) [12], catalase (CAT) [13], glutathione peroxidase (GSH-Px) [14], reduced glutathione (GSH) [15], thiobarbituric acid reactive substances (TBARS) [16], Hepatic G-6-Pase [17] and glycogen phosphorylase [18].Some pancreatic and liver tissues were stained with hematoxylin and eosin (H.E.) for histopathological examination. Other hepatic sections were stained using Periodic Acid Staining (PAS).
2.6. Statistical Analysis
- All results are expressed as mean ± Standard Error of Mean (SEM). Statistical analysis was performed using one-way Analysis of Variance (ANOVA) followed by Tukey’s post-hoc multiple comparison test using SPSS (version 16.0). The student’s ‘t’-test using SigmaPlot (version 8.0) was also performed. Values P<0.05 were considered statistically significant.
3. Results
3.1. Effect on Body Weight
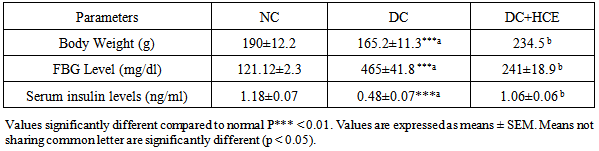
- All animals treated with alloxan in diabetic control group (DC) revealed a significant (P<0.05) loss in body weight. This was observed till the end of the study period (Table 1). Weight reduction, induced by diabetes in group (HCE), was cured by HCE treatment.
|
3.2. Effect on Fasting Blood Glucose Level, Serum Insulin
- Alloxan injection to DC and HCE groups resulted in a significant (P<0.05) increase in blood glucose level as compared to normal control group A, whereas there was a decrease in serum insulin levels. Treatment with H. clathratus hot water extract (HCE) for 30 days significantly reduced the glucose and enhanced insulin release) after 30 days of treatment (Table 1).
3.3. Effect of HCE on Activity of Hepatic glycogen Phosphorylase and Hepatic Glucose-6- phosphatase in Alloxan-induced Diabetic Rats
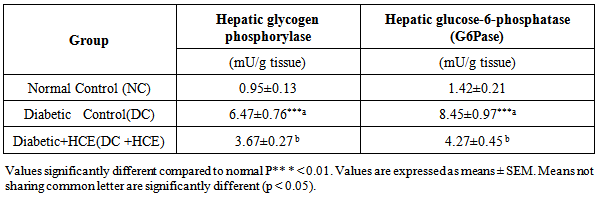
- Activity of hepatic glycogen phosphorylase and hepatic glucose-6- phosphatase increased significantly in diabetic control group (DC) compared to the normal control group (NC). However, treatment with H. clathratus hot water extract for 30 days significantly (P<0.05) decreased hepatic glycogen phosphorylase and hepatic glucose-6- phosphatase activities compared to DC (Table 2).
3.4. Effect on Serum Lipid Profile
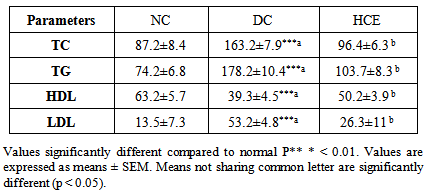
- Compared to normal control group (NC), the level of TC, TG, LDL serum cholesterol was significantly (P<0.05) increased whereas the level of HDL-cholesterol was significantly (P<0.05) reduced in untreated diabetic rats (DC) (Table 3). After treatment of group HCE with H. clatratus hot water extract for 30 days, the level of TC, TG, and LDL -cholesterol reduced significantly (P<0.05) whereas the level of serum HDL-cholesterol increased.
|
3.5. Anti-oxidative Status
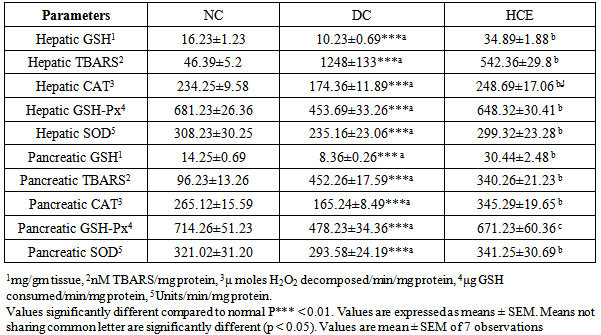
- It was observed that after inducing diabetes, the level of hepatic and pancreatic GSH (Glutathione reduced) content decreased significantly in diabetic control group (DC) compared to normal control group (NC). Treatment of group HCE with H. clathratus hot water extract for 30 days significantly (P<0.05) increased the tissue’s GSH level compared to DC (Table 4).
|
3.6. Effects of HCE on Alloxan-induced Histological Changes in Pancreatic Tissues
- The results of H. clathratus in histopathologic examination are shown in Fig. 1. As revealed in Fig. 1b, a clear decrease in the area occupied by the β-cells was observed in the pancreatic sections of alloxan-induced diabetic rats. Treatment with HCE showed a slight hypertrophy of Langerhans islets in pancreas compared to alloxan treated rats (Fig. 1c). This revealed the protective effect of HCE.
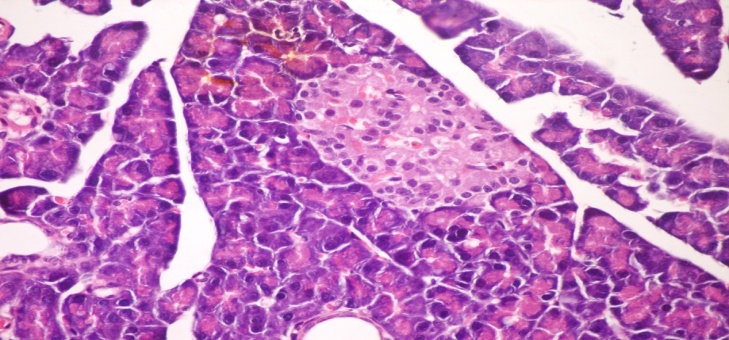 | Figure (1a). Pancreatic tissue of normal male albino rats. The pancreas is subdivided into pancreatic lobules. The exocrine portion of the pancreas consists of pancreatic acini, while the endocrine portion consists of islets of Langerhans (H&E x 400) |
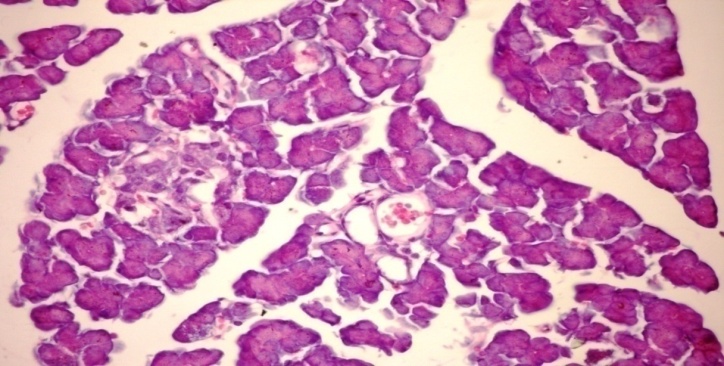 | Figure (1b). Pancreatic tissue of diabetic rats. Normal architecture of the islets is disrupted islets of Langerhans exhibited hydrophobic cells, necrotic cells, vacuolizations and irregular hyperchromic nuclei (H&E x 400) |
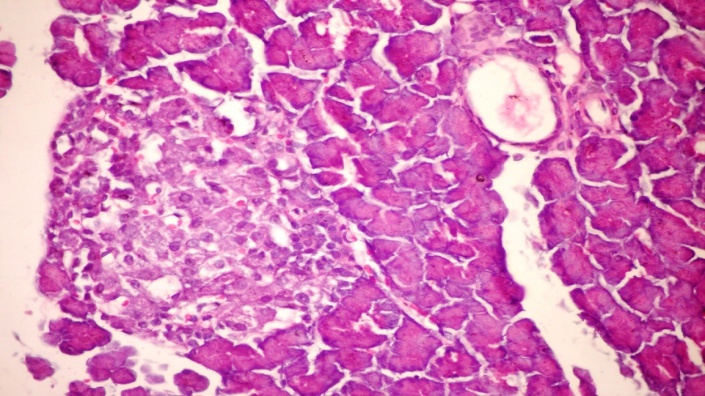 | Figure (1c). Pancreatic tissue of diabetic rats treated with HCE for 30 days consecutively) showing hypertrophy and vacuolations of β-cells of islets of Langerhans (H&E x 400) |
3.7. Effects of HCE on Alloxan-induced Histological Changes in Liver Tissue (H&E Stain)
- The results of H. clathratus in histopathologic examination are shown in Fig. 2. As revealed in Fig. 2b, Kupffer (check spelling, maybe K needs to be capitalized) cells activation, dilation and congestion of central vein and hepatic sinusoids were observed in the hepatic sections of alloxan-induced diabetic rats. Treatment with HCE showed improvement of in liver compared to alloxan treated rats (Fig. 2c), thus revealing the hepatic protective effect of HCE.Fig (2) Histological changes in Liver of diabetic rats (H&E).
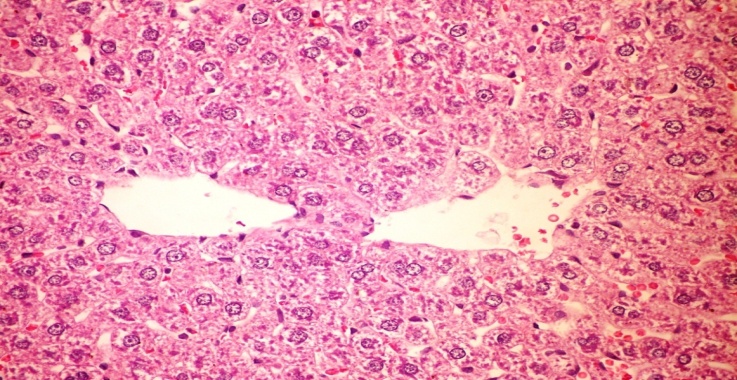 | Figure (2a). Liver of normal control rat showing the normal histological structure of hepatic lobule (H&E x 400) |
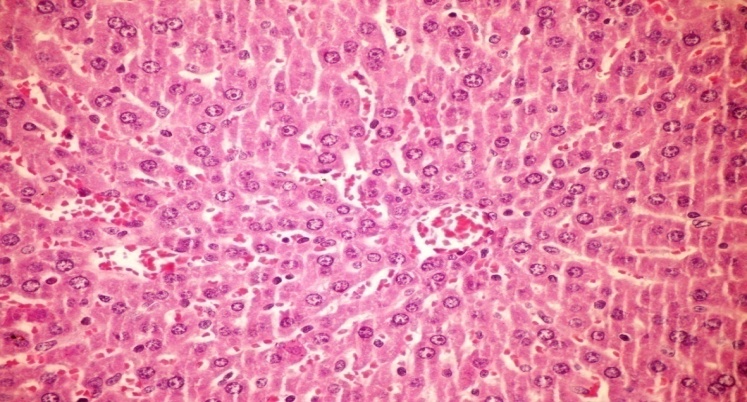 | Figure (2b). Liver of diabetic rat showing Kupffer cells activation, dilation and congestion of central vein and hepatic sinusoids (H&E x 400) |
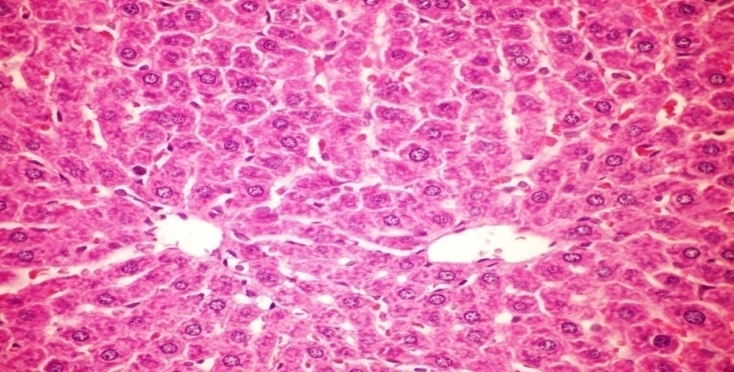 | Figure (2c). Liver section of diabetic rats treated with HCE (remove italics) (orally 400 mg/kg body weight of rats daily for 30 days consecutively) showing no histological changes (H&E x 400) |
3.8. Effects of HCE on Alloxan-induced Histological Changes in Liver (PAS)
- The results of H. clathrus in histopathological examination are shown in Fig. 3. As revealed in Fig. 3b, variable sized positive PAS glycogen particles were found distributed throughout the cytoplasm of the hepatic sections of alloxan-induced diabetic rats. Treatment with HCE revealed a moderate distribution of PAS particles throughout the hepatocytic cytoplasm in comparison to alloxan treated rats (Fig. 3c).
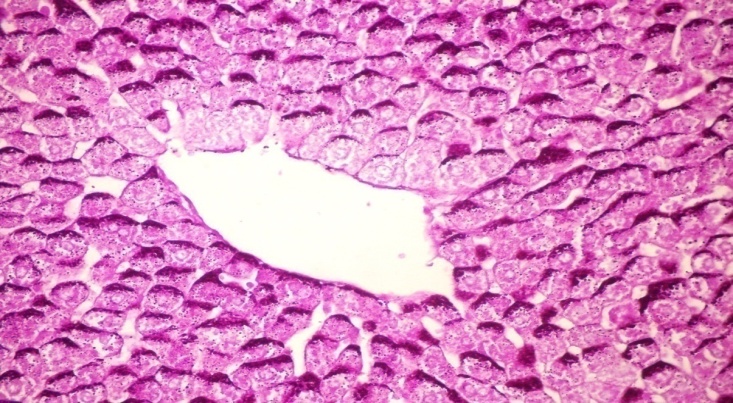 | Figure (3a). Liver tissue of normal control sample showing accumulation of reddish stained glycogen particles at one pole of hepatocytes (PAS stain x 400) |
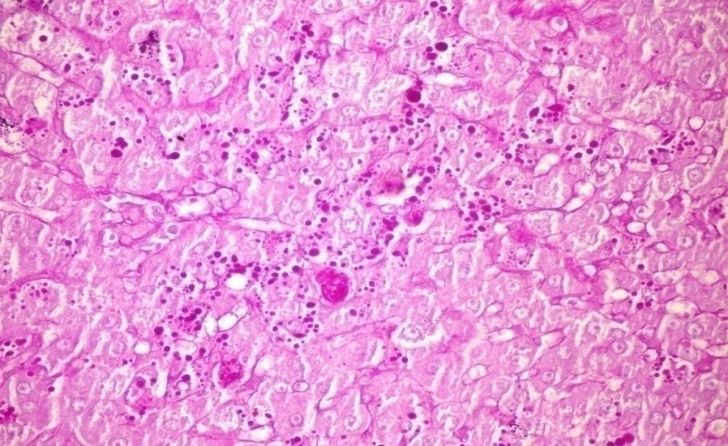 | Figure (3b). Liver tissue of alloxan-induced sample showing variable-sized positive PAS glycogen particles distributed throughout the hepatocytic cytoplasm (PAS stain x 400) |
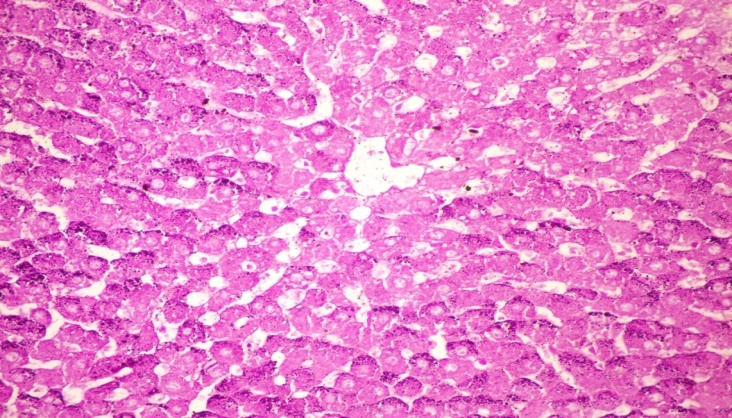 | Figure (3c). Liver tissue of diabetic sample treated with HCE (remove italics) (orally 400 mg/kg body weight of rats daily for 30 days consecutively) showing moderate PAs particles distributed throughout the hepatocytic cytoplasm (PAS stain x 400) |
4. Discussion
- Short-term exposure of β-cells to increasing glucose concentrations induces proliferation of β-cells in a concentration-dependent manner. That impairs β-cell secretory function. This glucotoxic effect is evident prior to apoptosis leading to a significant decrease in β-cell mass [19].Alloxan–induced diabetic rats exhibited severe glucose intolerance and metabolic stress as well as hyperglycemia due to a progressive oxidative stress interrelated with a decrease in endogenous insulin secretion and release. Hence treatment with antidiabetic drugs based on their pancreatic antioxidant activity might be a protective strategy for protecting β-cell [20].The present investigation indicated that a single dose of alloxan (150 mg/kg), intraperitoneally to adult male albino rats (190-210g) was suitable to induce histological changes to the morphology of the islets of Langerhans, evidenced by the resultant hypoinsulinemia and hyperglycemic state. The present dose as well as the observed histopathological and biochemical parameters agree with the previous literature [21].A gradual loss of β-cells due to apoptosis significantly hinders insulin production and inhibits cell viability. During apoptosis, cells shrink, chromatin condenses, DNA is cleaved into pieces at inter-nucleosomal regions. A proactive way to increase β-cell viability is to decrease apoptotic levels in order to retain the cell population and increase insulin production [22].Exposure of islets to alloxan shows significantly increased formation of peroxynitrite, NO and ROS with markedly elevated lipid peroxidation and reduced cell viability. Islets exposed to alloxan also show significantly increased mitochondrial membrane potential. Apparently, alloxan causes severe oxidative and cytotoxic stress to islets that is likely to compromise their insulin releasing capacity [23].Overproduction of ROS or exhaustion of anti-oxidants may cause oxidative stress. This is a major factor of defective insulin secretion and increases apoptotic potential of pancreatic cells [24]. Moreover, ROS produced by β-cell in response to metabolic stress affect mitochondrial structure and function and lead to β-cell failure. Specifically, ROS oxidize mitochondrial membrane phospholipids such as cardiolipin, which impairs membrane integrity and leads to cytochrome c release and apoptosis. In addition, ROS activate peroxidation of the mitochondrial membrane phospholipids, which results in proton leak leading to reduced ATP synthesis and content in β-cells [25].However, continuous treatment of diabetic animals with HCE for 30 days caused a significant reduction in FBG level. This antihyperglycemic action may be due to sulphated polysaccharides from marine algae that are known to be important free radical scavengers and antioxidants for the prevention of pancreatic oxidative damage, which is an important contributor in DM [26].The observed depletion of liver glycogen stores in diabetic rats is consistent with an earlier study [27] indicating that it could be due to the loss of glycogen-synthase activating system and/or increased activity of glycogen phosphorylase [28] since alloxan causes selective destruction of β-cells of the islets of langerhans resulting in a marked decrease of insulin levels. It is rational to believe that the activity of hepatic glycogen phosphorylase and hepatic glucose-6- phosphatase of diabetic animals will increased, as synthesis of glycogen depends on insulin for the influx of glucose. However, previous studies show that the reduced activity of glycogen phosphorylase and hepatic glucose-6- phosphatase are caused by insulin treatment [29].Administration of HCE for 30 days also showed a trend towards a significant increase in glycogen content when compared to diabetic rats, thus confirming its insulin potentiating effect to a certain extent because. Similarly, alloxan caused body weight loss to be regained to its above-initial values, by HCE treatment. This reflects an improved health state of HCE-treated animals. Histological observation of the pancreatic tissues further substantiates the claim that HCE has a protective function on pancreatic tissue.The characteristic features of diabetic dyslipidemia are a high plasma triglyceride, low HDL-cholesterol and an increased LDL-cholesterol particle concentration in the serum. Faulty glucose utilization causes hyperglycemia and mobilization of fatty acids from adipose tissue for energy purpose. The lipid changes associated with diabetes mellitus are attributed to the increased flux of free fatty acids into the liver, secondary to insulin deficiency/ resistance. This results in excess fatty acid accumulation in the liver, which is converted to triglycerides (ref). The impaired ability of insulin to inhibit free fatty-acid release leads to elevated hepatic VLDL (Very low density lipoprotein)-cholesterol production. The increased VLDL-cholesterol and triglyceride levels decrease the level of HDL-cholesterol and increase the concentration of small dense LDL-cholesterol particles by activation of lipoprotein lipase and lecithin acyl-cholesterol transferase. In our study, elevated levels of serum TC, TG and LDL cholesterol decreased HDL levels in alloxan-induced diabetic rats [31]. On other hand, the induction of diabetes by alloxan caused a decreased in body weight in the diabetic control rats. This was in accordance with [32] which suggest that this may due to alloxin inducing a catabolic effect on protein metabolism. It achieved this by retarding protein synthesis and stimulating protein degradation.However, treatment with HCE normalized all the lipid profile parameters. This antihyperlipidemic attribute of bark extract may also be attributed to sulphated polysaccharides, a major constituent of the extract, as sulphated polysaccharides enhance the negative charges of cell surfaces so as to effect the aggregation of cholesterol in blood, thus decreasing the cholesterol in serum [33].Earlier it has been explored that oxidative stress forms the foundation for the induction of multiple cellular pathways that can ultimately lead to both the onset and subsequent complications of DM. Diabetics and experimental animal models exhibit high oxidative stress due to persistent and chronic hyperglycemia. This depletes the activity of anti-oxidative defense systems and thus promotes de novo free radicalgeneration. Accumulated evidence from our laboratory has consistently shown that an early spike in GSSG (Glutathione disulfide) formation, typically within minutes of oxidant exposure, preceded oxidant-induced activation of mitochondrial apoptotic signaling and cell apoptosis hours later [34].In our study, the level of hepatic and pancreatic GSH was reduced in diabetic rats, which is consistent with an earlier report [35]. The decrease in tissue’s GSH content could be the result of decreased synthesis or increased degradation of GSH by oxidative stress that prevails during diabetes [36]. Furthermore, lipid peroxidation is one of the characteristic features of chronic diabetes. In the present study, along with decreased level of reduced GSH, a marked increase in the concentration of TBARS was also observed in liver and pancreas of diabetic mice, however, treatment of these animals with HCE decreased the elevated level of TBARS. Simultaneously, reduced GSH content was also increased significantly which indicates that HCE can either increase the biosynthesis of GSH and/or reduce oxidative stress that ultimately reduces the degradation of GSH. Similar results were also witnessed in an earlier study [37].Many more studies documented a pivotal role for H2O2 in the development of vascular dysfunction in pathological conditions, such as atherosclerosis, hypertension, and diabetes mellitus. It has also been speculated that, in the vessel wall, H2O2-mediated mechanisms may compensate for the loss of NO-mediated dilation during the development of various diseases [38].To prevent damage, it must be quickly converted into other, less dangerous substances. To this end, catalase is frequently used by cells to rapidly catalyze the decomposition of hydrogen peroxide into less reactive gaseous oxygen and water molecules. Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen.However, HCE therapy increased the activity of CAT and GSH-Px in hepatic and pancreatic tissues of treated diabetic animals. These findings can be complemented with the study of [39]. Reductone-associated sulphonic groups of polysaccharides can act as electron donors and can react with free radicals to convert these polysaccharides to more stable products and thereby terminate radical chain reactions [40].The increased activity of CAT, SOD and GSH-Px suggest a compensatory response (of oxidative stress as it reduces the endogenous H2O2 produced, thus diminishing the toxic effects due to this radical or other free radicals derived from secondary reactions [41] Moreover, the observed anti-oxidative effect of HCE may be due to synergistic action of various compounds present in the extract in contrast to the purified compound of HCE.Results of this study do not allow definite conclusion to be drawn on the mechanism of action of HCE in the experimental animal paradigms used. However, a number of investigators have shown that a host of various plant secondary metabolites possess hypoglycemic, hypotensive, anti-inflammatory and other pharmacological properties.
5. Conclusions
- From the results obtained, it can be concluded that HCE possess significant antihyperglycemic, antihyperlipidemic and anti-oxidative properties. . However, further studies are needed to investigate and elucidate the possible mechanism of action of the active ingredients of HCE, establish complete safety profiles and evaluate the potential value of HCE for the management of diabetes and hyperlipidemia. Moreover, additional parameters should also be studied for developing new drugs from this plant for managing diabetes and associated complications.
ACKNOWLEDGEMENTS
- The authors are grateful to Prof Dr Mohamed Bastawy, Professor of biochemistry for the financial support through the Department of Biochemistry, Faculty of science, Beni suef university.
References
| [1] | Park MK, Jung U, Roh C (2011): Fucoidan from marine brown algae inhibits lipid accumulation., Drugs, 9(8):1359-67. |
| [2] | Matsui T, Tanaka T, Tamura S, Toshima A, Miyata Y, Tanaka K (2007): Alphaglucosidase inhibitory profile of catechins and thioflavins., Journal of Agricultural and Food Chemistry, 55, 99–105. |
| [3] | Maiese K, Chong ZZ, Shang YC (2007): Mechanistic insights into diabetes mellitus and oxidative stress., Curr Med Chem; 14(16):1729-38. |
| [4] | Mellor KM, Ritchie RH, Delbridge LM (2010): Reactive oxygen species and insulin-resistant cardiomyopathy., Clin Exp Pharmacol Physiol, 37(2):222-8. |
| [5] | Matough FA, Budin SB, Hamid ZA, Alwahaibi N , Mohamed J (2012): The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J; 12(1):5-18. |
| [6] | Amin KA, Nagy MA (2009): Effect of carnitine and herbal mixture exract on obesity inducedby high fat diet in rats., Diabetol Metab Syndr. 16; 1(1):17. |
| [7] | Wang H, Chiu LCM, Ooi VEC, Ang Jr PO (2010): A potent antitumor polysaccharide from the edible brown seaweed Hydroclathrus clathratus., Bot. 53 265–274. (2012): Jayasooriya RG, Choi YH, Moon SK, Kim WJ. |
| [8] | Kim GY, Methanol extract of Hydroclathrus clathratus suppresses matrix metalloproteinase-9 in T24 bladder carcinoma cells by suppressing the NF-κB and MAPK pathways., Oncol Rep; 27(2):541-6. Viswanathaswamy AH, Koti BC, Gore A, Thippeswamy AH. |
| [9] | Kulkarni (2011): Antihyperglycemic and antihyperlipidemic activity of plectranthus amboinicus on normal and alloxan-induced diabetic rats. Indian J Pharm Sci., 73(2):139-45. |
| [10] | Trinder, P (1969): Plasma glucose was measured by standard methods.., Annals of Clinical Biochemistry, 6, 24–27. |
| [11] | Marschner I, Bottermann P, Erhardt F, Linke R, Löffler G, Maier V, Schwandt P, VogtW , Scriba PC "(1974) :"Group experiments on the radioimmunological insulin determination", Horm Metab Res. l;6(4):293-6. |
| [12] | Kakkar P, Das B, Viswanathan PN (1984): A modified spectrophotometric assay of superoxide dismutase., Ind J Biochem Biophys; 21: 130-32. |
| [13] | Lowry OH, Rosebrough, NJ, Farr AL, Randall RJ (1951) : Protein measurement with the folin phenol reagent., J Biol Chem; 193: 265-75. |
| [14] | Sinha AK (1972): Colorimetric assay of catalase., Anal Biochem; 47: 389-94. |
| [15] | Rotruck JT, Pope AL, Ganther HF, Swanson AB (1973): Selenium: Biochemical role as a component of glutathione peroxidase. Science; 179: 588-90. |
| [16] | Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction., Anal Biochem; 95: 351–58. |
| [17] | Begum N, Sam GPK, Shanmugasundaram KR (1978): Serum enzymes I human and experimental diabetes mellitus., Indian J Med Res.; 68:774-784. |
| [18] | Stalmans W, Hers HG (1975): The stimulation of liver glycogen phosphorylase b by AMP, fluoride and sulfate. A technical note of the specific determination of the α and β forms of liver glycogen phosphorylase., Eur j Biochem; 54:341-350. |
| [19] | Nagy MA and Ewais M (2014): Antidiabetic and antioxidative potential of Cystoseira myrica. American Journal of Biochemistry, Vol.4, N.4:(59-67). |
| [20] | Nagy MA and Mohamed SA (2014): Antidiabetic effect of Cloem Droserifolia (Cloemaceae). American Journal of Biochemistry, Vol.4, N.4:(68-75). |
| [21] | Nagy MA, Bastawy MA (2012): Renoprotective effects of Egyptian herbal formula during experimental diabetes. Access Scientific Reports. Vol (1) , N.9: (1-9). |
| [22] | Rabbani SI, Devi K, Khanam S (2010): Role of Pioglitazone with Metformin or Glimepiride on Oxidative Stress-induced Nuclear Damage and Reproductive Toxicity in Diabetic Rats., Malays J Med Sci. 17(1):3-11. |
| [23] | Lee E, Ryu GR, Ko SH, Ahn YB, Yoon KH, Ha H, Song KH (2011): Antioxidant treatment may protect pancreatic beta cells through the attenuation of islet fibrosis in an animal model of type 2 diabetes., Biochem Biophys Res Commun. 22; 414(2):397-402. |
| [24] | DU SC, Ge QM, Lin N, Dong Y , Su Q (2012): Ros-mediated lipopolysaccharide-induced apoptosis in ins-1 cells by modulation of bcl-2 and bax., Cell Mol Biol (Noisy-le-grand). 23; 58. |
| [25] | Ma ZA (2012): The Role of Peroxidation of Mitochondrial Membrane Phospholipids in Pancreatic β -Cell Failure., Curr Diabetes Rev. 1; 8(1):69-75. |
| [26] | Huang ZX, Mei XT, Xu DH, Xu SB Lv JY (2005): Protective effects of polysacchride of Spirulina platensis and Sargassum thunbeergii on vascular of alloxan induced diabetic rats., Zhongguo Zhong Yao Za Zhi. 30(3):211-5. |
| [27] | Hamden K, Carreau S, Jamoussi K, Miladi S, Lajmi S, Aloulou D, Ayadi F, Elfeki A (2009): 1Alpha, 25 dihydroxyvitamin D3: therapeutic and preventive affects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats., J Nutr Sci Vitaminol (Tokyo). 55(3):215-22. |
| [28] | Nagy MA, Bastawy MA, Abdel-Hamid NM (2012): Effects of Momordica Charantia on Streptozotocin-Induced Diabetes in Rats: Role of Insulin, Oxidative Stress and Nitric Oxide. Journal of Health Science, 2(2): 8-13. |
| [29] | El-Shenawy NS, Abdel-Nabi IM (2006): Hypoglycemic effect of Cleome droserifolia ethanolic leaf extract in experimental diabetes, and on non-enzymatic antioxidant, glycogen, thyroid hormone and insulin levels., Diabetologia Croatica; 35: 15-22. |
| [30] | Liu H, Gu L (2012): Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls., J Agric Food Chem. 8; 60(5): 1326-34. |
| [31] | Yadav UC, Moorthy K, Baquer NZ (2005): Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats., Mol Cell Biochem. 268(1-2):111-20. |
| [32] | Kamal Adel Amin, Ezzat Mohamed Awad and Mohamed A Nagy (2011): Effects of panax quinquefolium on streptozotocin-induced diabetic rats: role of C-peptide, nitric oxide and oxidative stress. Int.J.Clin.Exp.Med.1. 4(2):136-147. |
| [33] | Li YX, Kim SK (2011): Medicinal benefits of sulfated polysaccharides from sea vegetables. , Adv Food Nutr Res. 64:391-402. |
| [34] | Pias EK, Ekshyyan OY, Rhoads CA, Fuseler J, Harrison L , Aw TY(2003):Differential effects of superoxide dismutase isoform expression on hydroperoxide-induced apoptosis in PC-12 cells., J Biol Chem., 278:13294–13301. |
| [35] | Brito VB, da Rocha JB, Puntel GO, da Luz SC, Barbosa NB, de Carvalho NR, Folmer V (2011): Inhibition of δ-aminolevulinate dehydratase is not closely related to the development of hyperglycemia in alloxan-induced diabetic mice., Exp Toxicol Pathol. 63(5):443-51. |
| [36] | Nagy MA and Mohamed SA (2012): Momordica Charantia (Cucurbitaceae) methanolic extract alleviates alloxan-induced oxidative stress and β-cell damage in rat pancreas. Al-Azher assuit medical journal. Vol (10), N.3(173-196). |
| [37] | Hayashi S, Itoh A Isoda K, Kondoh M, Kawase M, Yagi K (2008): Fucoidan partly prevents CCl4-induced liver fibrosis., Eur. J. Pharmacol, 580, 380-384. |
| [38] | Kobayashi T, Kamata K (2002): Modulation by hydrogen peroxide of noradrenaline-induced contraction in aorta from streptozotocin-induced diabetic rat., Eur J Pharmacol 441: 83–89. |
| [39] | Hlawaty H, Suffee N, Sutton A, Oudar O, Haddad O, Ollivier V, Laguillier-Morizot C, Gattegno L, Letourneur D, Charnaux N(2011): Low molecular weight fucoidan prevents intimal hyperplasia in rat injured thoracic aorta through the modulation of matrix metalloproteinase-2 expression., Biochem. Pharmacol, 81, 233–243. |
| [40] | Aisa Y, Miyakawa Y, Nakazato T, Shibata H, Saito K, Ikeda Y (2004): Fucoidan induces apoptosis of human HS-Sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways, American Journal of Hematology, 78, 7–14. |
| [41] | Hu JF, Geng MY, Zhang JT, Jiang HD (2001): An in vitro study of the structure-activity relations of sulfated polysaccharides from brown algae to its antioxidant effect., J Asian Nat Prod Reports 3:353-358. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML