-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(4): 68-75
doi:10.5923/j.ajb.20140404.02
Antidiabetic Effect of Cleome droserifolia (Cleomaceae)
1Chemistry department, Faculty of science, Beni-Suef University, Egypt
2Biohemistry department, Faculty of Medicine, Azher university, Assuit, Egypt
Correspondence to: Nagy MA, Chemistry department, Faculty of science, Beni-Suef University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Ethnopharmacological relevance: Cleomedroserifolia is used in egyptien traditional medicine for treatment of diabetes mellitus. The aim of the present study was to evaluate the possible protective effects of Cleomedroserifolia methanolic extract (CDE) against pancreas β-cells’ damage and antioxidant defense systems in alloxan induced diabetic rats. Materials and methods: Experimental diabetes was induced by a single dose of alloxan (150 mg/kg) administered by intraperitoneal way. The oxidative stress was measured by tissue MDA levels, reduced glutathione (GSH) content and by enzymatic activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) in pancreas. Biochemical observations were further substantiated with histological examination of pancreas. Results: The increase in blood glucose and MDA levels with the decrease in GSH content and in enzymatic activities were the salient features observed in diabetic rats. Administration of CDE (0.31g/kg bw/day, orally) for 30 days caused a significant reduction in blood glucose and MDA levels in alloxan treated rats when compared with diabetic rats. Furthermore, diabetic rats treated with CDE showed a significant increase in the activities of both enzymatic and non-enzymatic antioxidants when compared to those of diabetic rats. Degenerative changes of pancreatic β-cells in alloxan treated rats were minimized to near normal morphology by administration of CDE as evidenced by histopathological examination. Conclusion: Results clearly indicate that Cleomedroserifolia treatment exerts a therapeutic protective nature in diabetes by decreasing oxidative stress and pancreatic β-cells’ damage which may be attributed to its antioxidative potential.
Keywords: Cleomedroserifolia, Diabetes mellitus, Oxidative stress, Antidiabetic, Antioxidant
Cite this paper: Nagy MA, Mohamed SA, Antidiabetic Effect of Cleome droserifolia (Cleomaceae), American Journal of Biochemistry, Vol. 4 No. 4, 2014, pp. 68-75. doi: 10.5923/j.ajb.20140404.02.
Article Outline
1. Introduction
- The increasing incidence of diabetes represents an enormous socio-economic burden in the developing countries. The World Heath.Organization estimates that over 300 million people worldwide will have (Diabetes mellitus) DM by the year 2025 with alarming proportions from developing countries [31].DM is a chronic disease caused by inherited or acquired deficiency in insulin secretion and by decreased responsiveness of the organs to secrete insulin. There are two main forms of diabetes. Type 1 diabetes is due primarily to autoimmune-mediated destruction of pancreatic islet beta-cells, resulting in dramatic insulin deficiency. Its frequency (~ 10%) is low relative to Type 2 diabetes (T2D), which accounts for over 90% of cases. T2D is characterized by abnormal insulin secretion, associated with varying degrees of insulin resistance [23].β cells normally compensate insulin resistance by secreting more amounts of insulin to maintain the glucose homeostasis. In the course of time, however, this beta cell function gets impaired leading to deterioration in glucose homeostasis and subsequent development of impaired glucose tolerance and frank diabetes. There occurs only a relative insulin deficiency as the day-long circulating insulin concentrations in diabetic patients that are almost comparable or slightly elevated in absolute terms to the values in normal individuals [8].β cell dysfunction results from prolonged exposure to high glucose, ROS or a combination of both. β-cells are particularly sensitive to ROS because they are low in free-radical quenching (antioxidant) enzymes such as CAT, GPx and SOD. Therefore, the ability of oxidative stress to damage mitochondria and (markedly blunt insulin secretion is not surprising [20].As a consequence, blood glucose levels rise, passing from normal to impaired glucose tolerance first, and to overt diabetes eventually. Notably, deterioration of diabetes control and insulin secretary function occurs with years in diabetic patients despite insulin resistance remains stable. Thus, β-cell dysfunction is central to the development of diabetes, possibly due to a combination of decreased beta-cell mass and insulin secretion defects [21].Alloxan, a β-cytotoxin, has demonstrated severe physiological and biochemical derangements of the diabetic state. The alloxan rats exhibited severe glucose intolerance and metabolic stress as well as hyperglycemia due to a progressive oxidative insult interrelated with a decrease in endogenous insulin secretion and release [1].Herbal remedies that stem from egyptien traditional medecine hold a great promise against DM. The dried herb of Cleome droserifolia (Forssk.) Del., is a plant of the Cleomaceae family. It is present in the deserts, especially the Eastern desert, Red Sea region, Sinai, Gebel .Its decoction of leaves and stems is widely used by the Bedouins of the southern Sinai for the treatment of diabetes [7].Methanolic extract of leaves and stems for Cleome droserifolia (CDE) is rich in Bioactive compounds as flavanoids, flavonol glycosides, alkaloids, tannins and Steroids as shown in fig (1). So far as plant phenolics constitute one of the major groups of compounds acting as primary antioxidants or free radical terminators, Flavonoids as one of the most diverse and widespread group of natural compounds are probably the most important natural phenolics as they possess radical scavenging properties [25].
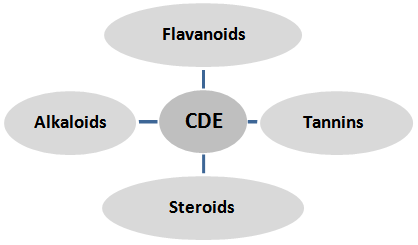 | Figure 1. Princiciple phytochemical components in CDE |
2. Materials and Methods
2.1. Chemicals
- Alloxan monohydrate was purchased from sigma Fine chemicals. All other chemicals used for this study were of analytical grade and obtained from Stanbio Laboratory USA Kits. Kits for the estimation of total cholesterol, triglyceride and HDL-cholesterol were purchased from Diamond Diagnostic Egypt.
2.2. Plant Material
- The aerial parts of were collected from wildly growing populations on El-Ketar mountain, Hurghada, Egypt were authenticated by Dr. Hany Ezzat Khalil (Pharmacognacy Department, Faculty of Pharmacy, El Minia, Egypt.
2.3. Preparation of Plant Extract
- The freshly collected leaves and stem part of Cleome droserifolia were washed with distilled water and air-dried under the control conditions and powdered. The powdered plant material was percolated with petroleum ether to remove fatty substances then further exhaustively extracted with of 80% methanol for 3 days. The extract was filtered, concentrated on rotavapour and then freeze-dried under high vacuum (1.3 Pa) and at temperature of - 40± 2°. The extract will be dissolved in 0.5g Carboxy methyl cellulose (0.5w/v)° for oral administration.
2.4. Animalsl
- 30 Healthy male albino rats (5-7 months old, weighing (190-210 g) were procured from Faculty of Agricultural, El Minia University, Egypt. They were housed under standard laboratory conditions of light (12:12 h L: D cycle), temperature (23 ± 2°C) and relative humidity (55 ± 5%). The animals were provided standard rat pellet feed and tap water ad libitum. Maintenance and treatment of all the animals was done in accordance with the principles of Institutional Animal Ethics Committee constituted as per the directions of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Egypt.
2.5. Induction of Experimental Diabetes
- For the present study, animals were divided into following 3groups:NC (normal control), DC (diabetic control) and CDE (diabetic + Cleome droserifolia methanolic extract treated). After fasting for 18 hours, rats of group DC, CDE were made diabetic by a single intra-peritoneal injection of alloxan monohydrate, 150 mg/kg body wt, freshly dissolved in normal saline [40]. Subsequent to alloxan administration the rats had free access to food and water and were provided with 50% glucose solution to drink overnight to counter drug induced hypoglycemic shock. One week after alloxan injection, the fasting blood glucose (FBG) concentration was determined by means of one touch ultra glucometer (Johnson & Johnson Company, USA) and compatible blood glucose strips [39]. Rats showing fasting blood level greater than 140 mg/dl were considered diabetic and selected for treatment with methanolic extrcat of Cleome droserifolia (CDE) (0.31g/kg body wt.). The CDE were administered orally, once in a day for 30 days.
2.6. Experimental Design
- The rats were randomly divided into four groups of seven animals each:Group I (NC): normal control rats, received vehicle solution NaCl (1 ml/kg, intraperitoneal way) for 30 days.Group II (DC): diabetic control rats, received alloxan in single dose (150 mg/kg bw, intraperitoneal way).Group III (DC+CDE): CDE-treated diabetic rats received by oral way, 3 days after alloxan treatment, 0.31g /kg bw of CDE extract for 30 days.On the last day of experiment, animals were sacrificed and blood samples were collected without heparin for biochemical estimations. Some pancreas were removed, cleaned and washed in ice-cold normal saline solution for biochemical analysis.
2.7. Biochemical Assays
- Biochemical estimations in blood and serum insulin:Fasting blood glucose (FBG) concentration of all the four experimental groups was determined by glucometer during different phases of the experiment by withdrawing blood from the tail vein. Serum insulin was assayed in the Radioactive Isotopes Unit, Central Department of Scinentificial Analysis and Test, National Research Center (Dokki, Giza) by radioimmunoassay kits of DPC (Diagnostic Products Corporation, Los Angeles, USA) [coat-A-count] [22].For estimating serum lipid profile, serum was isolated from the blood collected by cardiac puncture under mild ether anesthesia from overnight fasted rats on day 30th of CME treatment and serum total cholesterol(TC) and triglyceride (TG) using diagnostic kits (Erba Mannheim Cholesterol kit, Transasia Bio-Medicals Ltd., Daman). Results were expressed in mg/dl.Biochemical estimation in tissue homogenates: Pancreas were removed, freed from adhering tissues and washed with ice-cold normal saline solution (0.9%). Weight of all the organs was taken only after drying the tissue. 1 g tissue was homogenized in 10 ml of 0.2 M tris-HCl with the help of homogenizer. The homogenate was filtered and then centrifuged at 10,000 rpm for 20 minutes at 4°C. The supernatant obtained was used for estimation of SOD [11], catalase CAT [17], glutathione peroxidase GSH-Px [36], reduced glutathione GSH [34]and thiobarbituric acid reactive substances [28].
2.8. Histopathological Examination of Pancreas
- Some pancreas were cleaned and fixed in 10% neutral buffered formalin solution. After dehydration in graded ethanol solutions and in toluene, they were embedded in paraffin. Sections of 3–5µm thickness were stained with hematoxylin and eosin (H.E.) for histopathological examination.
2.9. Statistical Analysis
- Statistical analysis was carried out using Graph Pad Instat software (version 3, ISS-Rome, Italy). Groups of data were compared with ANOVA, followed by Tukey-Kramer (TK) multiple comparisons post-test. Values of P < 0.05 were regarded as significant. Data were expressed as mean ± standard error (SEM).
3. Results
3.1. Effects of Cleome Droserifolia Methanolic Extracts on Body Weight, Hyperglycemia and Hypoinsulinemia Induced by Alloxan
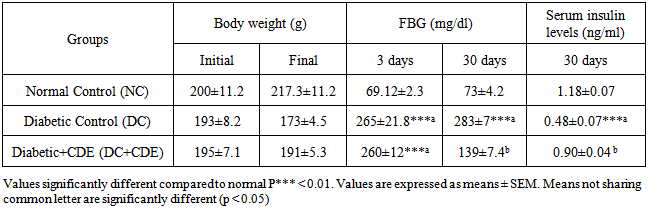
- Table 1 depicts the initial and final body weight, the levels of FBG and plasma insulin in control and experimental groups of rats. Diabetic rats (alloxan) presented at the end of treatment a significant loss of body weight as compared to control ones which gained a significant weight. In addition, FBG was significantly increased by and those of serum insulin were significantly decreased in the diabetic rats when compared to the control group.
|
3.2. Effects of Cleome droserifolia Methanolic Extract on Lipid Profile of Alloxan-induced Diabetic Rats
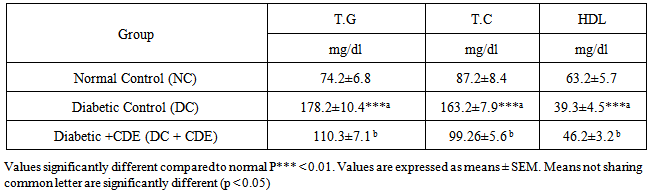
- The effects of Cleome droserifolia methanolic extract on lipid parameters are presented in Table 2. Our results showed that the administration of alloxan increased significantly total cholesterol (TC) and triglycerides (TG) levels after 30 days of treatment, in comparison to control rats. The administration of Cleome droserifolia methanolic extract countered the significant rise in the levels of the parameters that illustated by significant increse in HDL-cholesterol level.
|
3.3. Effects of Cleome droserifolia Methanolic Extract on Alloxan-induced Lipid Peroxidation and GSH Content
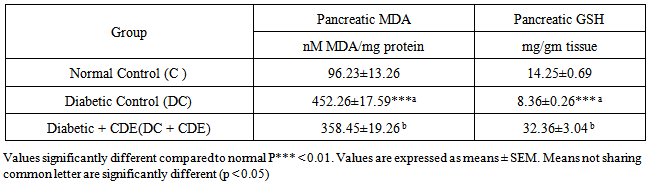
- Table 3 represents the levels of MDA and GSH in pancreatic tissue of the control and experimental rats. The diabetic rats showed a significant increase in MDA levels and a decrease in GSH levels when compared to those of control group.
|
3.4. Effects of Cleome droserifolia Methanolic Extract on Alloxan-induced Changes in the Antioxidant Enzyme Activities
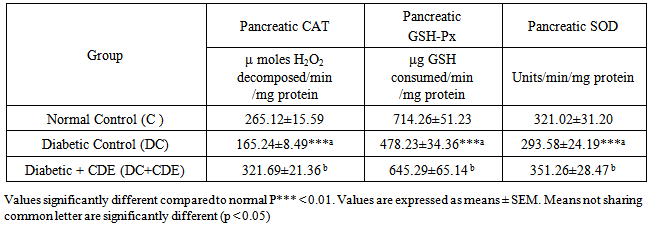
- Table 4 The activities of enzymatic antioxidants such as SOD, CAT and GPx in the control and experimental groups of rats. The activities of these enzymatic antioxidants were significantly decreased in the diabetic rats when compared to those of control group. Oral administration of CDE to the diabetic rats showed a significant increase in the activities of SOD, CAT and GPx.
|
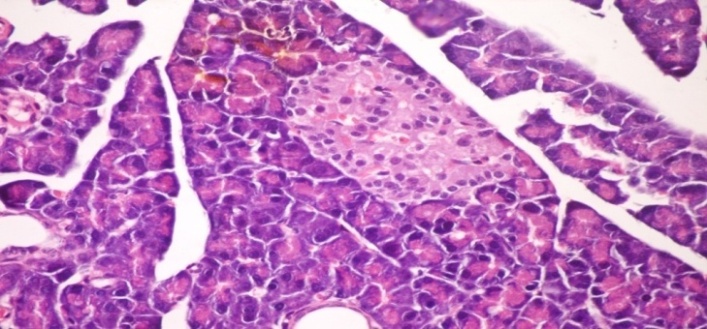
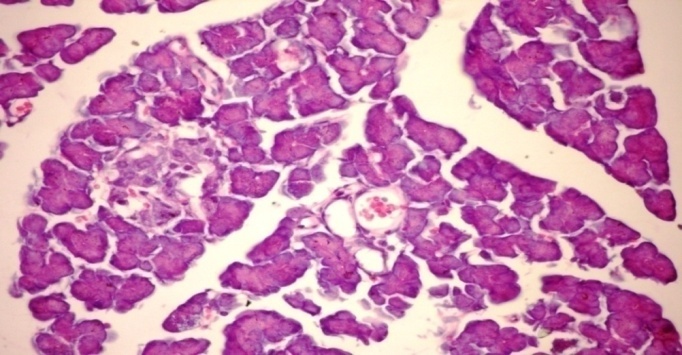
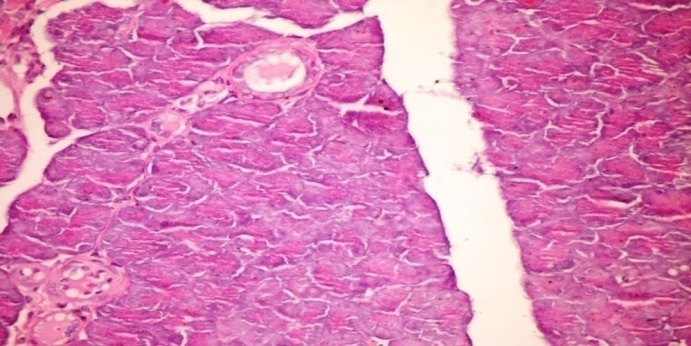
3.5. Effects of Cleome droserifolia Methanolic Extract on Alloxan-induced Histological Changes in Pancreas
- The results of Cleome droserifolia in histopathologic examination are shown in Fig. 2. As revealed in (Fig. 2B), a clear decrease in the area occupied by the β cells was observed in the pancreatic sections of alloxan-induced diabetic rats. Treatment with CDE showed a slight hypertrophy of Langerhans islets and hyperplasia in pancreas compared to alloxan treated rats (Fig. 2C) revealing the protective effect of CDE.
4. Discussion
- Glucose is the key physiological regulator of insulin secretion; indeed, short-term exposure of β-cells to increasing glucose concentrations induces proliferation in a concentration-dependent manner. In addition to its effect on β-cell turnover, hyperglycemia also impairs β-cell secretary function. This glucotoxic effect is evident before apoptosis leads to a significant decrease in β-cell mass [5]. Alloxan–induced diabetic rats exhibited severe glucose intolerance and metabolic stress as well as hyperglycemia due to a progressive oxidative insult interrelated with a decrease in endogenous insulin secretion and release [37].Treatment with antidiabetic drugs based on their pancreatic antioxidant activity might be a protective strategy for protecting β-cell due to disproportionate generation of free radicals [10].The present investigation indicated that a single dose of alloxan (150 mg/kg) intraperitoneally to adult male albino rats (190-210g) was suitable to induce histological changes of the islets of Langerhans characterized appearance, hypoinsulinemia and hyperglycemic state. The present dose as well as the observed histopathological and biochemical manifestations agree with previous literature [14]. A gradual loss of β-cells due to apoptosis significantly hinders insulin production and inhibits cell viability. During apoptosis, cells shrink; chromatin condenses; DNA is cleaved into pieces at inter nucleosomal regions. A proactive way to increase β-cell viability is to decrease apoptosis level in order to retain the cell population and increase insulin production [33].Exposure of islets to alloxan shows significantly increased formation of peroxynitrite, NO and ROS with markedly elevated lipid peroxidation and reduced cell viability. Islets exposed to alloxan also show significantly increased mitochondrial membrane potential. Apparently, alloxan causes severe oxidative and cytotoxic stress to islets that is likely to compromise their insulin releasing capacity [15].Overproduction of ROS or exhaustion of antioxidants may cause oxidative stress which is a major factor of defective insulin secretion and increases apoptosis of pancreas [6]. Moreover, ROS produced by β-cell in response to metabolic stress affect mitochondrial structure and function and lead to β-cell failure. Specifically, ROS oxidize mitochondrial membrane phospholipids such as cardiolipin, which impairs membrane integrity and leads to cytochrome c release and apoptosis. In addition, ROS activate UCP2 via peroxidation of the mitochondrial membrane phospholipids, which results in proton leak leading to reduced ATP synthesis and content in β-cells [19].However, continuous treatment of diabetic animals with CDE for 30 days caused a significant reduction in FBG level accompanied with significant enhancement in serum insulin in accordance with previous study [27]. CDE has a hypoglycemic effect through potentiation of peripheral, hepatic insulin sensitivity and diminishing intestinal glucose absorption [18]. On other hand, Flavonoids, the major active constituent in CDE are potential antidiabetic agents because they exert multiple actions that are both hypoglycemic (insulinomimetic action) and antihyperglycemic (insulin secretagogue). Also, flavonoid-enriched extract from efficiently inhibited a-glucosidase activity and may inhibit the non-Na+dependent facilated diffusion of monosaccharides in intestinal epithelial cells [2]. Consequently, the parallel concentrative Na + dependent transport ATPase for monosaccharides gains efficiency [4].Similarly, alloxan caused body weight loss was also regained to its above-initial values by CDE treatment, which reflects an improved health of CDE treated animals. The histological observation of the pancreatic tissues further substantiates the claim that CDE has a protective nature on pancreatic tissue.The characteristic features of diabetic dyslipidemia are a high plasma triglyceride concentration, low HDL-cholesterol concentration and increased concentration of small dense LDL-cholesterol particles. Faulty glucose utilization causes hyperglycemia and mobilization of fatty acids from adipose tissue for energy purpose. The lipid changes associated with diabetes mellitus are attributed to increased flux of free fatty acids into the liver secondary to insulin deficiency/ resistance. This results in excess fatty acid accumulation in the liver, which is converted to triglycerides. The impaired ability of insulin to inhibit free fatty-acid release leads to elevated hepatic VLDL-cholesterol production. The increased VLDL-cholesterol and triglyceride levels decrease the level of HDL-cholesterol and increase the concentration of small dense LDL-cholesterol particles by activation of lipoprotein lipase and lecithin acyl-cholesterol transferase In our study, elevated levels of serum TC, TG and LDL cholesterol and decreased HDL cholesterol concentration in alloxan-induced diabetic rats are in accordance with previous study [42a].On other hand, Induction of diabetes by alloxan resulted in loss of body weight in the diabetic control rats in accordance with previous study [16] that may due to catabolic effect on protein metabolism by retarding protein synthesis and stimulating protein degradation.However, treatment with CDE causes significant decrease in the serum levels of triglycerides, total cholesterol and enhancement of body weight in accordance with previous literature [30].Flavonoids in CDE inhibit the activity of cAMP - dependent protein phosphokinase, the consequence is that the cAMP concentration increases and that phosphorylation of the Hydroxy methyl glutaryl-CoA reductase, but endogenous cholesterol production is diminished. In addition, the flavonoids can interact with the enzyme protein phosphatase, which liberates the aliphatic phosphoesters from Hydroxy methyl glutaryl-CoA-CoA HMG-CoA reductase, thus restoring the activity of this. Thus, flavonoids inhibit HMG-CoA reductase by a dual mechanism [35].Earlier it has been explored that oxidative stress forms the foundation for the induction of multiple cellular pathways that can ultimately lead to both the onset and subsequent complications of DM. Diabetics and experimental animal models exhibit high oxidative stress due to persistent and chronic hyperglycemia, which thereby depletes the activity of antioxidative defense system and thus promotes de novo free radicals generation [24]. GSH oxidation is a major contributor to cell apoptosis mediated by oxidants. Accumulated evidence from our laboratory has consistently shown that an early spike in GSSG formation, typically within minutes of oxidant exposure, preceded oxidant-induced activation of mitochondrial apoptotic signaling and cell apoptosis hours later [32]. In our study, the level of pancreatic GSH was reduced in diabetic rats, which is consistent with an earlier report [3]. The decrease in tissue’s GSH content could be the result of decreased synthesis or increased degradation of GSH by oxidative stress that prevails during diabetes. Furthermore, lipid peroxidation is one of the characteristic features of chronic diabetes [42b]. In the present study, along with decreased level of reduced GSH, a marked increase in the concentration of MDA was also observed in pancreas of diabetic mice; however, treatment of these animals with CDE decreased the elevated level of MDA. Simultaneously, reduced GSH content was also increased significantly which indicates that the CDE can either increase the biosynthesis of GSH and/or reduce the oxidative stress that ultimately reduces the degradation of GSH. Similar results were also witnessed in an earlier study [29]. Catalase is a common enzyme found in nearly all living organisms. Its functions include catalyzing the decomposition of hydrogen peroxide to water and oxygen. One of the most commonly used ROS for neuronal oxidative stress preconditioning is H2O2. It is formed by the dismutation of superoxide (O2) spontaneously or enzymatically in a reaction that is catalyzed by superoxide dismutase (SOD). H2O2 is less reactive compared to other ROS, easily crosses membranes, and diffuses from its original site of production.Many more studies documented a pivotal role for H2O2 in the development of vascular dysfunction in pathological conditions, such as atherosclerosis, hypertension, and diabetes mellitus. It has also been speculated that, in the vessel wall, H2O2-mediated mechanisms may compensate for the loss of NO-mediated dilation during the development of various diseases [12].To prevent damage, it must be quickly converted into other, less dangerous substances. To this end, catalase is frequently used by cells to rapidly catalyze the decomposition of hydrogen peroxide into less reactive gaseous oxygen and water molecules. Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen.However, Oral administration of CDE causes significant decrease in Pancreatic MDA and significant enhancement in level of pancreatic GSH and the activity of pancreatic CAT, pancreatic GSH-Px and pancreatic SOD. These findings can be complemented with previous study of [13]. Flavonoids usually contain one or more aromatic hydroxyl groups, which actively scavenge free radicals and are responsible for the antioxidant activity. On other hand, lipophilicity of flavenoids were factors deciding the antioxidant property, most probably affecting the depth of incorporation of a compound in the lipid phase of membrane [9].Results of this study do not allow definite conclusion to be drawn on the mechanism of action of CDE in the experimental animal paradigms used. However, a number of investigators have shown that a host of various plant secondary metabolites possess hypoglycemic, anti-inflammatory and other pharmacological properties [41].As mentioned earlier, CDE is also known to contain various secondary metabolites. Therefore, it is not unreasonable to speculate that some of these chemical compounds are presumably responsible for imparting the antihyperglycemic, antihyperlipidemic and antioxidative properties to CDE.From the results obtained, it can be concluded that CDE possess significant antihyperglycemic, antihyperlipidemic and pancreatic antioxidative properties. Hence, apart from controlling hyperglycemia it would also be beneficial in the alleviation of associated diabetic complications including the prevention of the development of atherosclerosis and other coronary artery diseases. However, further studies are needed to investigate and elucidate the possible mechanism of action of the active ingredients, establish complete safety profiles and evaluate the potential value of CDE for the management of diabetes and hyperlipidemia in the clinic.Moreover, additional parameters such as assay of fructosamine, HbA1c, C-peptide etc should also be studied. This may prove helpful for developing new drugs from this plant for managing diabetes and associated complications.
ACKNOWLEDGEMENTS
- The authors are indebted to Prof. Dr. Nabil Mohie El dien, Professor of biochemistry, Faculty of Pharmacy, El Minia University, Egypt for his kind support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML