-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(3): 46-51
doi:10.5923/j.ajb.20140403.02
Preparation and Biological Evaluation of 125I Labeled 6-Iodo-2-(4-methoxyphenyl)-1Hbenzo [d] Imidazole as Imaging Agent
1Nutrition Depart. College of Designs and Home Economy, Qassim University, Bldg. 3897, Buraydah 52383-8314, Saudi Arabia
2Inshas Cyclotron Facility, Physics Department, Nuclear Research Center, Atomic Energy Authority, Cairo 13759, Egypt
Correspondence to: Atteyat A. Labib, Nutrition Depart. College of Designs and Home Economy, Qassim University, Bldg. 3897, Buraydah 52383-8314, Saudi Arabia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
6-Iodo-2-(4-methoxyphenyl)-1H benzo [d] imidazole is synthesized as example of benzoimidazole derivative. It could be labeled with auger emitter 125I successfully with yield about 94.5%. The labeled product was evaluated by paper chromatographic method and paper electrophoresis.Then the reaction parameters studied were amount of benzoimidazole derivative, pH of the reaction mixture, reaction time, temperature, and amount of oxidizing agents to optimize the conditions for the labeling of benzoimidazole derivative and to obtain a high radiochemical yield of the 125I benzoimidazole derivative. In a reaction vial of 1.0 ml capacity with a Teflon septum and screw cap, 200 μCi of sodium [125I] iodide, 0.1 mg of substrate, 50 μl of CAT as oxidizing agent at room temperature for 15 min, a maximum radiochemical yield of (94.5%) was obtained. The biodistribution data showed substantial uptake of 4.54±0.10 (%ID/g±SD) in the brain at 5 min post-injection. The biological distribution in normal mice indicates that radioiodinated 125I benzoimidazole derivative is a novel agent for brain imaging.
Keywords: 125I benzoimidazole derivative, Electrophoresis, Imaging agent, Tissues distribution
Cite this paper: Atteyat A. Labib, Preparation and Biological Evaluation of 125I Labeled 6-Iodo-2-(4-methoxyphenyl)-1Hbenzo [d] Imidazole as Imaging Agent, American Journal of Biochemistry, Vol. 4 No. 3, 2014, pp. 46-51. doi: 10.5923/j.ajb.20140403.02.
Article Outline
1. Introduction
- In recent years, different imidazole and benzoimidazole drugs have been found to be associated with several biological activities such as antiparasitic, antifungal, anti-inflammatory, and antibacterial activities [1-7]. Some imidazole and benozimidazole derivatives have surface activity and when used in high concentrations are able to damage membranes [8, 12]. This situation is independent on the culture medium and growth rate. Imidazoles can interact directly with the lipid bilayer of the plasma membrane, probably by binding to the unsaturated fatty acids [13, 17].Alzheimer’s disease (AD) is a slowly progressive and fatal neurodegenerative brain disorder associated with progressive episodic memory loss and decrease of cognitive functions and characterized by the presence of amyloid plaques and neurofibrillary tangles [18-19]. As the number of affected people, as well as the economical and social impact, will increase in the future due to the increasing average life span, the search for a radioactive tracer which allows in vivo diagnosis of AD in an early stage has become an important research object during the last years [20-25]. Developing β – amyloid imaging probes is currently an emerging field of research. The basic requirements for suitable probes include (i) good penetration of the blood–brain barrier, (ii) selectively binding or labeling β-amyloid plaques, and (iii) displaying clear and contrasting signals between plaques and non-plaques.Based on these requirements, several promising agents with the backbone structure of DDNP, thioflavin-T, and Congo Red have been synthesized and evaluated for use in vivo as probes to image β-amyloid plaques in AD brain. Recently, a number of groups have reported new β-amyloid binding probes without the basic structures of DDNP, thioflavin-T and Congo Red. Most of these probes have two aromatic rings. Among them, 1,4-diphenyltriazole and 2,5-diphenylthiophene derivatives have triazole and thiophene between two benzene rings, respectively, and it has been shown that they have tolerance for binding to Ab aggregates [26, 27]. This paper describes the study carried out for the preparation of iodinated benozimidazole derivative using CAT as oxidizing agent and the influence of various reaction parameters and conditions on radioiodination efficiency were investigated and optimized in order to maximize the radiochemical yield.
2. Experimental
2.1. Materials
- All chemicals were purchased from Merck Co. and all other reagents were of analytical grades. Chloramine-T [N- chloro-p toluene sulfonamide salt (CAT)] from Aldrich. Thin layer chromatography (TLC) aluminum sheets (20 9 25 cm) SG-60 F254 (Merck). Na125I (185 MBq/5 ml) in diluted NaOH, pH 7–11 was purchased from Institute of Isotopes, Budapest, Hungary.
2.2. Equipments
- The 1H-NMR spectra were recorded on a Varian Gemini 200 NMR Spectrometer at 300 MHz with TMS as a standard, Mass spectrum was performed by a shimadzu Gc-MS-QP, 100 Ex (shimadzu, Japan), Faculty of Science, Cairo University. Radioactivity was measured by means of a gamma-counter (Nucleus Model 2010) connected with a well type NaI (Tl) crystal.
2.3. Animals
- Female Swiss Albino mice weighing 20–25 g were purchased from the Institute of Eye Research Cairo, Egypt. The animals were kept throughout the experimental period at room temperature (22 ± 2)℃ with a 12 h on/off light schedule. Standard food and water were allowed to mice all over the experiments.
2.4. Chemistry
2.4.1. 6-Bromo-2-(4-methoxyphenyl)-1Hbenzo[d]imidazole (I)
- A mixture of 4-bromobenzene-1,2-diamine (187 mg,1.0 mmol), 4-methoxy benzaldehyde (149mg, 1.0 mmol), and Na2S2O5 (190mg,1.0 mmol) dissolved in 10 ml of dimethylformamide (DMF) was heated to reflux for 2h. Ice water (70 ml) was added, and the precipitate formed was collected by filtration, washed with water and dried under vacuum to obtain 246 mg of as a yield of 81.2%. 1H NMR (400 MHz, CD3OD) δ 8.01 (d, J=8.8 Hz, 2H), 7.71 (s, 1H), 7.47 (d, J=8.2 Hz, 1H), 7.34 (d, J=8.5 Hz, 1H), 7.09 (d, J=8.8 Hz, 2H), 3.88 (s, 3H). m/z calcd for C14H11BrN2O 302.01 found 303.09 (M+H+).
2.4.2. 2-(4-Methoxyphenyl)-6-(tributylstannyl)-1Hbenzo [d]imidazole (II)
- A mixture of (I) (126 mg, 0.4 mmol), (Bu3Sn)2, 0.4 ml and (Ph3P)4 Pd (47 mg, 0.04 mmol) in a mixed solvent (10 ml, 1:1 dioxane/Et3N) was stirred under reflux for 10 h. The solvent was removed, and the residue was purified by TLC (hexane: ethyl acetate=2:1) to give 35 mg of II was obtained in a yield of 27.2%. 1H NMR (400 MHz, CD3OD) δ 8.03 (d, J=9.0 Hz, 2H), 7.68 (s, 1H), 7.57 (d, J=7.8 Hz, 1H), 7.29 (d, J=7.8 Hz, 1H), 7.08 (d, J=9.0 Hz,2H), 3.88 (s, 3H), 1.73–0.79 (m, 27H). MS: m/z calcd for C26H38N2OSn 514.20; found 515.31 (M+H+).
2.4.3. 6-Iodo-2-(4-methoxyphenyl)-1Hbenzo [d] imidazole (III)
- 25 mg of (II) was used, and 15 mg of (III) was obtained in a yield of 45.5%. 1H NMR (400 MHz,CD3OD) δ 8.01 (d, J=8.9 Hz, 2H), 7.91 (s, 1H), 7.52 (dd, J=8.4, 1.6 Hz, 1H), 7.37 (d, J=8.4 Hz, 1H), 7.09 (d, J=8.9Hz, 2H), 3.88 (s, 3H). HRMS m/z (EI+): calcd for C14H11IN2O 349.9916; found 349.9909.
3. Labeling Procedure
- Radiolabeled benozimidazole derivative was synthesized by iododestannylation reaction with 125I under oxidative conditions in the presence of CAT. 125I affords the ability to use a high specific activity iodide without adding carrier iodine. Briefly, In a reaction vial of 1.0 ml capacity with a Teflon septum and screw cap, 50 μg of CAT was added to a mixture of a benozimidazole derivative (0.1 mg/100 μl in ethanol), 200 μCi of sodium [125I] iodide (specific activity 2200 Ci/mmol), and 100 μl of 1 M HCl in asealed vial. The reaction was allowed to proceed at room temperature and the reaction mixture was shaken by electric vortex and left at room temperature for 5, 15, 30, 45 and 60 min. A 50 μl of saturated sodium metabisulfite solution (10 mg/mL H2O) was added to decompose the excess of iodine [12, 13] in order to quench the reaction by reducing it to iodide (I-). The reaction mixture was extracted with ethyl acetate (3×1 ml) after neutralization with 10 mg of sodium bicarbonate. The combined extracts were evaporated dry. The residues were dissolved in 100 μl of EtOH and the radiochemical yield of the product was determined by paper chromatography and paper electrophoresis. The influences of various reaction parameters and conditions on radioiodination efficiency, such as the amount of oxidizing agents (CAT), concentration of substrate, pH of the reaction, reaction time and reaction temperature, were investigated and optimized in order to maximize the radiochemical yield.
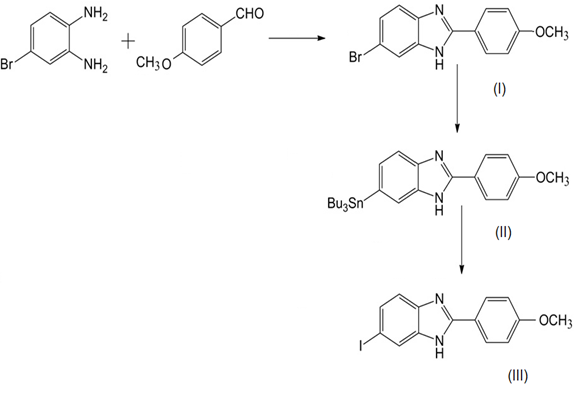 | Figure 1. Scheme Preparation of 6-Iodo-2-(4-methoxyphenyl)-1Hbenzo [d] imidazole (III) |
3.1. Estimation of 125I Benzoimidazole Derivative Yield
- Radiochemical yield and purity of 125I benzoimidazole derivative were determined by paper chromatographic method using strips of Whatman paper. On paper sheet (1 cm width and 13 cm length), 1–2 µL of the reaction mixture was placed 2cm above the lower edge and allowed to evaporate spontaneously. For development a fresh mixture of chloroform: ethanol (9:1 v/v) was used. After complete development, the paper sheet was removed, dried, and cut into strips, each strip is 1 cm width, and then each strip was counted in a well type ɤ-counter. Radiochemical yield was further confirmed by paper electrophoresis. On Whatman paper sheet (2 cm width and 47 cm length), 1–2 µL of the reaction mixture was placed 12 cm above the lower edge and allowed to evaporate spontaneously. Electrophoresis is carried out for 80 min at voltage of 300 V using a normal saline (0.9% w/v NaCl solution) as electrolytes source solution. After complete development, the paper was removed, dried, and cut into strips, each strip is 1 cm width, and then the strip was counted in a well type ɤ-counter. The percentage of radiochemical yield was estimated as the ratio of the radioactivity of 125I benzoimidazole derivative to the total activity multiplied by 100 [26].
3.2. Estimation of in Vitro Stability of 125I Benzoimidazole Derivative
- The reaction mixture was left at room temperature for 72 h and 1–2 µL samples were taken from it at different time intervals. The radiochemical yield and purity of the samples were measured by paper chromatography and paper electrophoresis.
3.3. Estimation of the Partition Coefficient for 125I Benzoimidazole Derivative
- The partition coefficient was determined by mixing 125I benzoimidazole derivative with equal volumes of 1-octanol and phosphate buffer saline (PBS) (0.05 M at pH 7.4) in a centrifuge tube. The mixture was vortexed at room temperature for 1 min and then centrifuged at 5,000 rpm for 5 min. Subsequently 100 µL samples from the 1-octanol and aqueous layers were pipetted into other test tubes and counted in a gamma counter. The measurement was repeated three times. The partition coefficient value was expressed as log p [20].
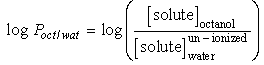
4. Results and Discussion
- The labeled compound was estimated by paper chromatography where radioiodide (I-) remained near the origin (Rf = 0–0.1), while the 125I benzoimidazole derivative moved with the solvent front (Rf = 0.8). Radiochemical purity was further confirmed by paper electrophoresis where the free radioiodide and 125I benzoimidazole derivative moved to different distances away from the spotting point towards the anode (distance from spotting point = 14 and 10 cm, respectively) depending on the molecular weight of each one. Results of radiochemical yield from the two separation methods (paper chromatography and paper electrophoresis) are nearly the same. The influence of various reaction parameters and conditions on radioiodination efficiency was investigated and optimized in order to maximize the radiochemical yield.
4.1. Effect of CAT Amount
- Labeling yield was increased with the increase of CAT content till 50 µg, which is the optimum content. Above 50 µg no significant change in the labeling yields (Table 1).
4.2. Effect of Substrate Amounts
- The dependence of radiochemical yield on the amount of benzoimidazole derivative is shown in table. 2 The reaction was performed at different benzoimidazole derivative amounts (25–100µg). The radiochemical yield of 125I- benzoimidazole was small at low substrate concentration (for instance, at 25 µg the labeling yield was 80.4 ± 0.31%), and by increasing benzoimidazole derivative concentration, the labeling yield was increased (for instance the labeling yield is 94.5 ± 0.40 % at 100 µg).
|
4.3. Effect of pH of the Reaction
- The effect of pH of the reaction mixture on the labeling of benzoimidazole derivative was studied by changing the pH value in the range from 3 up to 9 using buffers to adjust pH at the desired value the data representing in (Table 3). The experiment was performed using 10 µg of benzoimidazole derivative. CAT amount used was 50 µg, while other factors were kept constant. The experiment was repeated using 100 lL of each buffer at different values of pH. The labeling yield for each pH value of the reaction mixture was measured. It was observed that, the maximum value of the labeling yield achieved when the pH value ranged from 4 to 7. As low and higher pH the labeling yield decreases.
|
4.4. Effect of Reaction Time
- The labeling yield is strongly dependent on reaction time in the range from 1 to 30 min. It is clear from table 4 that the yield is significantly increased with increasing the reaction time. The results indicate that the reaction is very fast. After 15 min the maximum radiochemical yield (94.2 ± 0.3 %) was obtained.
|
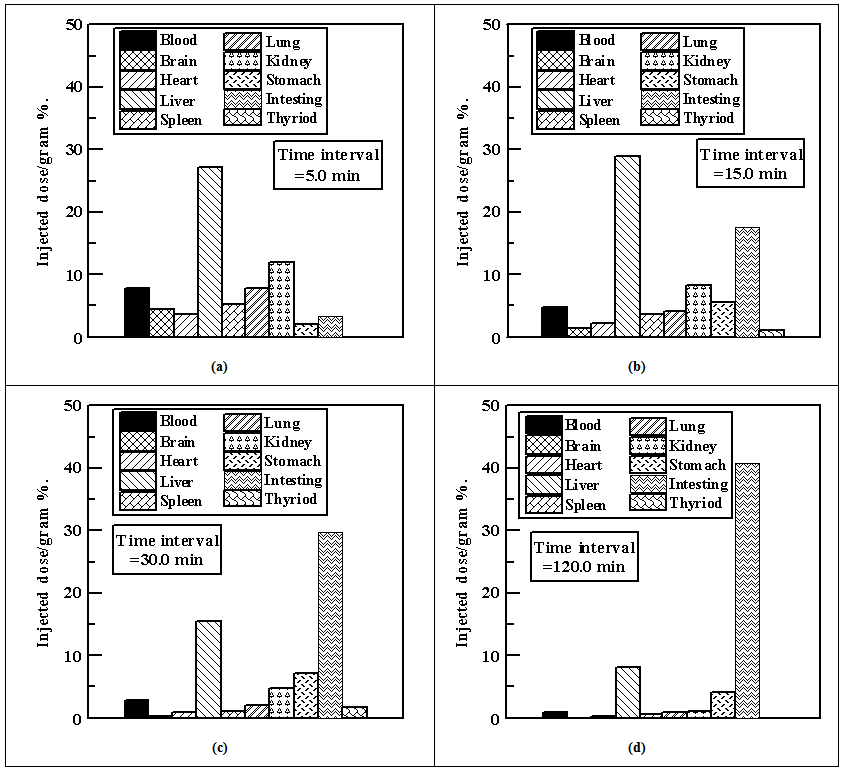 | Figure 2. Injected dose / gram for different organs body fluids and for the time interval equals: (a) 5.0 min, (b) 15.0 min, (c) 30.0 min, and (d) 120.0 min |
4.5. In Vitro Stability of 125I Benzoimidazole Derivative
- In vitro stability of 125I benzoimidazole derivative was studied in order to determine the suitable time for injection to avoid the formation of the undesired products that result from the radiolysis of the labeled compound. These undesired radioactive products might be accumulated in non-target organs. The results of stability showed that the 125I benzoimidazole derivative is stable up to 48 h as shown in table 5.
|
4.6. Partition Coefficient of 125I Benzoimidazole Derivative
- The partition coefficient value was 2.35 ± 0.11, showing that 125I benzoimidazole derivative is relatively lipophilic and can cross the blood–brain barrier.
4.7. Biodistribution Studies
- The Biodistribution experiments were performed in normal mice (average weight, about 25 g). A saline solution (100 μl, 5% EtOH) containing 125I benzoimidazole derivative (1 μCi) was injected directly into the tail vein. The mice were sacrificed at various time points post-injection. The organs of interest were removed and weighed, and the radioactivity was measured with gamma counter (Nucleus Model 2010) connected with a well type NaI (Tl) crystal. The percent dose per gram of wet tissue was calculated by a comparison of the tissue counts to suitably diluted aliquots of the injected material.
4.7.1. Biodistribution of 125I-benzoimidazole Derivative
- The biodistribution pattern of 125I benzoimidazole derivative is shown in figure 2. 125I benzoimidazole derivative was injected in normal mice via intravenous route and was distributed all over the body organs and fluids. All radioactivity levels are expressed as average percentage of injected dose per gram (% ID/g ± SD). The bio distribution data showed substantial uptake of 4.54±0.10 (% ID/g ± SD) in the brain at 5 min post-injection. After this time, point radioactivity dropped to 0.09±0.01 at 120 min post-injection. The intestine indicates that excretion of 125I benzoimidazole derivative occurs mainly through the hepatobiliary pathway. The low radioactivity located in the thyroid gland indicates that the iodocompounds are stable against in vivo deiodination. The high accumulation of the 125IMPBI in lungs is attributed to the fact that lungs function as reservoir for antidepressants with high affinity to the serotonin transporter [27]. The bio distribution data showed substantial lung uptake of (4.18±0.54% ID/g ± SD) at 15 min post injection. The maximum brain uptake of 125I benzoimidazole derivative (4.54±0.10) is higher than that of currently used radiopharmaceuticals for brain imaging, 99mTc-ECD and 99mTc-HMPAO which have maximum brain uptake of 4.7 and 2.25%, respectively [15]. These preliminary results strongly suggest that benzoimidazole derivative which is labeled with 125I could be used as a novel agent for brain imaging. The high uptake of 125I benzoimidazole derivative in stomach also enforces us to do more research about the possibility of using it as agent for stomach imaging and treatment comparing it with currently used agents for that purpose.
5. Conclusions
- 125I benozimidazole derivative was synthesized in high yield and excellent quality using of 200 μCi of sodium [125I] iodide, 0.1 mg of substrate, 50 μl of CAT as oxidizing agent at room temperature for 15 min, a maximum radiochemical yield of 94.5 ± 0.40% was obtained. The In biodistribution studies fast clearance from the blood indicated a promising tracer for β-amyloid plaques imaging.
References
| [1] | Selkoe, D. J. Physiol. Rev. 2001, 81, 741. |
| [2] | Blennow, K.; de Leon, M.; Zetterberg, H. Lancet 2006, 368, 387. |
| [3] | Graczyk, P. P., Khan, A., Bhatia, G. S., Palmer, V.,Medland, D. & Numata, H. The neuroprotective action of JNK3 inhibitors based on the6, 7- dihydro-5H-pyrrolo [1, 2-a] imidazoles scaffold. Bioorg Med Chem Lett, 2005, 15, 4666-4670. |
| [4] | Uaucu, R., Karaburun, N. G. & Isikdag, I. Synthesis and analgesic activity of some 1-benzyl-2-substituted-4, 5-diphenyl-1Himidazole erivatives. Farmaco, 2001, 56, 285-290. |
| [5] | Antolini, M., Bozzoli, A., Ghiron, C., Kennedy, G., Rossi, T. & Ursini, A. Analogues of 4,5 bis(3,5-dichlorophenyl)-2-trifluoromethyl-1Himidazole as potential antibacterial agents. Bioorg Med Chem Lett, 1999, 9, 1023-1028. |
| [6] | Simonetti, G., Baffa, S. & Simonetti, N. Contact imidazole activity against resistant bacteria and fungi. Int J Antimicrob Ag, 2001, 17, 389-393. |
| [7] | Duquenoy, P. & Ruysschaert, J.M. Interaction between lipids and miconazole sulfosalicylate and econazole sulfosalicylate. Eur Bull Drug Res, 1993, 2, 129-134. |
| [8] | Dahiya R, Kumar A, Yadav R Molecules 2008,13:958–976. |
| [9] | Siddiqui N, Andalip BS, Ali R, Afzal O, Akhtar MJ, Azad B, Kumar R J Pharm Bioall Sci 2011, 3(20):194–212. |
| [10] | Kumar JR Pharmacophore. 2010, 1(3):167–177. |
| [11] | Bart A, Jansen J, Zwan JV, Dulk H, Brouwer J, Reedijk J. J Med Chem 2001, 44:245– 249. |
| [12] | Shinkai H, Ito T, Iida T, Kitao Y, Yamada H, Uchida I .J Med Chem 2000, 43:4667– 4677. |
| [13] | Klimesova V, Svoboda M, Waisser K, Pour M, Kaustova. J Farmaco 1999,54:666– 672. |
| [14] | Zhuang ZP, Kung MP, Hou C, Plossl K, Skovronsky D, Gur TL, et al. IBOX(2-(4′-dimethylaminophenyl)-6-iodobenzoxazole): a ligand for imaging amyloid plaques in the brain. Nucl Med Biol 2001; 28:887–94. |
| [15] | Qu WC, Kung MP, Hou C, Benedum TE, Kung HF. Novel styrylpyridines as probes for SPECT imaging of amyloid plaques. J Med Chem 2007; 50:2157–65. Bhatia MS. |
| [16] | Kung MP, Hou C, Zhuang ZP, Zhang B, Skovronsky D, Trojanowski JQ, et al. IMPY: an improved thioflavin-T derivative for in vivo labeling of beta-amyloid plaques. Brain Res 2002; 956:202–10. |
| [17] | Maria GM, Valeria F, Daniele Z, Luciano V, Elena B .J Farmaco 2001,56:587–592. |
| [18] | Mulani AK, Choudhari PB, Ingale KB, Bhatia NM. Int J Drug Discov. 2009, 1:1–9. |
| [19] | Muthal N, Ahirwar J, Ahriwar D, Masih P, Mahmdapure T, Sivakumar T Int J Pharm Tech Res 2010, 2(4):2450–2455. |
| [20] | Labib.A.A. Isotopic exchange method for preparation of iodine-125-n-(1-p-nitrobenzaldhydephenyl)-2-(2-iodophenyl)-4-(4-nitrobenzilidene)-imidazolin-5-one and its biological evaluation. International Journal of Basic and Applied Sciences, 2014, 3 (2) 78-85. |
| [21] | Rovelet-Lecrux, A.; Hannequin, D.; Raux, G.; Le Meur, N.; Laquerrière, A.; Vital, A.; Dumanchin, C.; Feuillette, S.; Brice, A.; Vercelletto, M.; Dubas, F.; Frebourg, T.; Campion, D. Nat. Gent. 2006, 38. |
| [22] | Brouwers, N.; Sleegers, K.; Engelborghs, S.; Bogaerts, V.; Serneels, S.; Kamali, K.; Corsmit, E.; De Leenheir, E.; Martin, J.-C.; De Deyn, P. P.; Van Broeckhoven, C.; Theuns, J. Brain 2006, 129, 2994. |
| [23] | Chao, Q.; Sprankle, K. G.; Grotzfeld, R. M.; Lai, A. G.; Carter, T. A.; Velasco, A. M.;Gunawardane, R. N.; Cramer, M. D.; Gardner, M. F.; James, J. A.; Zarrinkar, P. P.;Patel, H. K.; Bhagwat, S. S. J. Med.Chem. 2009, 52, 7808. |
| [24] | Kung, H. F.; Choi, S. R.; Qu, W. C.; Zhang, W.; Skovronsky, D. J. Med. Chem. 2010,53, 933. |
| [25] | Neirinckx RD, Canning LR, Piper IM, Nowotnik DP, Pickett RD, Holmes RA, Volkert WA, Forster AM, Weisner PS, Marriott JA, Chaplin SB J Nucl Med. 1987, 28:191–202. |
| [26] | Qu, W.; Kung, M. P.; Hou, C.; Oya, S.; Kung, H. F. J. Med. Chem. 2007, 50,3380. |
| [27] | Chandra, R.; Kung, M. P.; Kung, H. F. Bioorg. Med. Chem. Lett. 2006, 16, 1350. |
| [28] | Labib A. A. Synthesis, radioiodination and biodistribution evaluation of 5-(2-amimo-4-styryl pyrimidine-4-yl)-4- methoxybenzofuran-6-ol. Asia Oceania Journal of Nuclear Medicine and Biology. (2013). 1 32, 32-38. |
| [29] | Zhuang ZP, Kung MP, Wilson A, Lee CW, Plossl K, Hou C, et al. Structure-activity relationship of imidazo[1,2-a] pyridines as ligands for detecting β-amyloid plaques in the brain. J Med Chem 2003;46: 237–43. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML