-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(2): 29-34
doi:10.5923/j.ajb.20140402.03
Fatty Acid and Amino Acid Profile of Emperor Moth Caterpillar (Cirina forda) in Paikoro Local Government Area of Niger State, Nigeria
Yohanna B. Paiko1, John O. Jacob1, Simon O. Salihu1, Bukar E. N. Dauda1, Mohammed A. T. Suleiman1, Helmina O. Akanya2
1Department of Chemistry, Federal University of Technology, PMB 65, Minna, Nigeria
2Department of Biochemistry, Federal University of Technology, PMB 65, Minna, Nigeria
Correspondence to: John O. Jacob, Department of Chemistry, Federal University of Technology, PMB 65, Minna, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
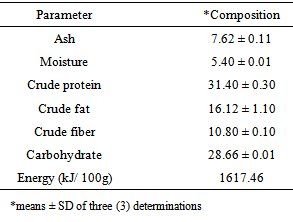
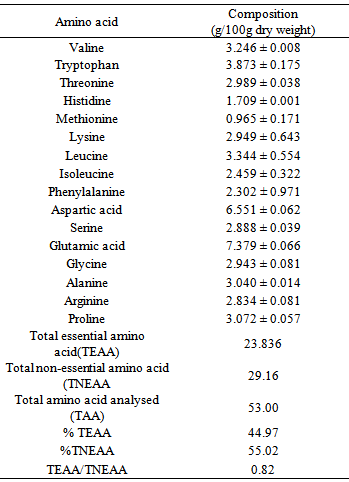
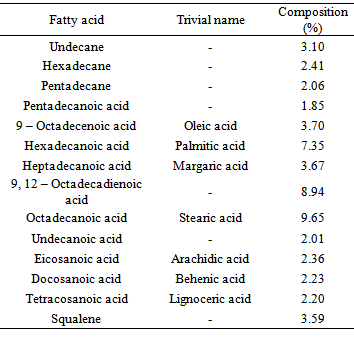
The proximate, amino acid, fatty acid and mineral compositions of emperor moth caterpillar (Cirina forda) were investigated. A high protein and lipid contents of 31.40 ± 0.3 and 16.12 ± 1.1 (% dry weight) respectively were recorded. The insect was found to contain varying proportions of essential and non-essential amino acids and also saturated and unsaturated fatty acids. An ash content of 7.62 ± 0.11 (% dry weight) was obtained containing high proportions of calcium, sodium, potassium, magnesium and phosphorus (32.24, 45.50, 65.04, 67.31, and 111.00 mg/100g) respectively. Moderate amounts of iron, manganese and zinc were also detected. A K/Na ratio of 1.53 ± 0.04 obtained suggests that this insect could be a potential source of diet for the management of hypertension. The high protein and carbohydrate contents also suggest that the emperor moth caterpillar could serve as an alternative source of protein and other nutrient supplements in human and animal diet.
Keywords: Emperor Moth Caterpillar, Fatty Acid, Amino Acid, Proximate Composition, Mineral Composition
Cite this paper: Yohanna B. Paiko, John O. Jacob, Simon O. Salihu, Bukar E. N. Dauda, Mohammed A. T. Suleiman, Helmina O. Akanya, Fatty Acid and Amino Acid Profile of Emperor Moth Caterpillar (Cirina forda) in Paikoro Local Government Area of Niger State, Nigeria, American Journal of Biochemistry, Vol. 4 No. 2, 2014, pp. 29-34. doi: 10.5923/j.ajb.20140402.03.
Article Outline
1. Introduction
- Insects are widely spread all over the world; many of them are well known and appreciated for their characteristics [1]. Entomophagy (the habit of eating insects as food) is a well established custom in many parts of the world [2], and many edible insects have become a food resource that is being tapped by various communities. In Europe, Asia, Australia and Africa, people feed on different stages of various insects. Early records by Ene [3], showed that the ancient Greek- Parthians and Nasamines ate locust and grasshoppers as food. The French feed on the abdomen of the beetle Rhizotrogus assimilis. In Nigeria several species of insects are prominent items of commerce in the village markets and many families make fairly good living from selling insects [4].This has contributed significantly to the reduction of protein deficiencies [5]. The caterpillar of the pallid emperor moth (Cirina forda) is of the order, Lepidoptera and family, Saturniidae. It is an insect pest of Butyrospermun paradoxum, the shearbutter tree and is widely accepted as a food source and is also an important item of commerce in many Nigerian states such as Oyo, Kwara, Kogi, Niger, Kaduna and Benue [5]. The larvae of this insect are processed into the dried form and consumed as a delicacy served as snacks or as an essential ingredient in vegetable soups along with carbohydrate food in Southern Nigeria and many homes in Africa [6, 7, 8]. The nutritional qualities of edible insects have been studied by various authors including Mbata and Chidumayo [9], and Ogbadu [10]. They have been found to contain protein and abundant fat calories. Ande [11] recorded high protein content and 21.45% ether proportion in C. forda but the quality was not reported. He concluded that they may be a good nutritional source to man. In Niger State, only a few of these edible insects have been analyzed for their nutritional content. The quality of the nutrients in most of the popularly consumed insects is fragmentary and inadequate. Lack of such information is standing in the way of the utilization of these relatively rich protein and lipid sources for the dietary requirements of man. This study therefore attempts to evaluate the proximate composition, mineral content, the fatty acid and amino acid profiles of emperor moth caterpillar from Paikoro Local Government, Niger State, and to compare these values with reports on other plant and animal protein and lipid sources. This will provide a baseline data and a reference point for the study of nutritional potentialities of other edible insects which will encourage greater exploitation to meet other malnutrition needs in the rural communities.
2. Materials and Methods
2.1. Sample Collection, Preparation and Analysis
- Fresh samples of the emperor moth caterpillar (Cirina forda) were handpicked from the crowns of Shear butter trees in ten different farmlands between the months of May to August, 2010 in Tutungo Village, Paikoro Local Government, Niger State. The samples were washed and sun-dried for 72 hours, milled into powder ready for analysis.
2.2. Proximate Analysis
- The moisture, ash and crude fiber contents were determined by the methods of Association of Official Analytical Chemists (AOAC) [12]. Total nitrogen (%N) was determined by the micro- Kjeldahl method [12], and crude protein was obtained by using a Nitrogen Protein conversion factor of 6.25. The oil (lipid %) content was obtained by extracting 5 g of the powdered sample with chloroform: methanol (2: 1) mixture as described by the modified Folch method [13]. The solvent was evaporated on a rotary evaporator and the oil obtained was dried in an oven at 60 oC to remove all traces of solvent. Percentage Nitrogen Free Extract (Carbohydrate) was obtained by difference applying the formula:100 % - (% protein + % lipid + % ash + % fibre)All determinations were in triplicate.
2.3. Mineral, Fatty acid and Amino Acid Analyses
- Sodium and Potassium were determined using Gallenkamp flame analyzer, while Calcium, Magnesium, Iron, Manganese, Zinc and Copper were determined using Buch 211 Atomic Absorption Spectrophotometer. Phosphorus content was determined using the phosphovanado molybdate colorimetric method and the results read on JENWAY 6100 spectrophotometer. The oil in the sample was extracted using chloroform: methanol (2:1) mixture, as described by modified method by Folch [13]. The extracted oil was hydrolysed and the fatty acids converted to their fatty acid methyl derivatives and their concentrations determined using GC/MS. The defatted samples were hydrolysed and after derivatization, the amino acid profile was obtained using WATERS 1525 binary HPLC coupled with multiwavelength fluorescence detector. Duplicate determinations were carried out in each case.
3. Results and Discussion
- The result of the proximate chemical composition of emperor moth caterpillar is presented in Table 1.
|
|
|
|
4. Conclusions
- In this study fatty acids and amino acid profiles of emperor moth caterpillar (Cirina forda) were investigated using GC/MS and HPLC respectively. Proximate and mineral analyses were also carried out using methods of AOAC and flame analyzer and AAS respectively. The results obtained show that the insect is a good source of protein, essential amino acids, fatty acids and other nutrient supplements. Consumption of this unpopular insect is encouraged so as to reduce the pressure on other conventional protein sources among the poorer sector of the population and to alleviate the problem of nutrient deficiency.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML