-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(2): 25-28
doi:10.5923/j.ajb.20140402.02
Ameliorative and Protective Effect of Omega 3 – Fatty Acid on Testicular Lipid Concentration in Ethanol- Induced Wistar Rats
Adetona Mutiat Olutope1, Solanke Adebayo Solomon2, Amusan Kabiru Ayantayo2
1Faculty of Pure and Applied Sciences, Southwestern University Nigeria, P.M.B. 2088, Okun –Owa, Ijebu – Ode, Ogun –State
2Faculty of Basic Medical Science, College of medicine, University of Lagos, Idi – Araba, Lagos, Lagos -state
Correspondence to: Adetona Mutiat Olutope, Faculty of Pure and Applied Sciences, Southwestern University Nigeria, P.M.B. 2088, Okun –Owa, Ijebu – Ode, Ogun –State.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Ethanol is a molecule soluble in both water and lipids. It can diffuse into all tissues of the body and affect the vital organs. However, an increased intake of ethanol is known to increase the levels of lipids (hyperlipidemia). Although significant progress has been made in understanding the pathogenesis of ethanol testicular damage, alternative therapies are needed to supplement the existing synthetic drugs. Nutritional antioxidants have been reported to play an important role in cellular antioxidative defense mechanisms. Research has made it known that Omega -3 fatty –acid can suppress leukocyte activity, cell proliferation, inflammatory cytokine synthesis, natural killer cell activity, antibody synthesis and macrophage surface membrane protein expression, and they also act as free radical scavengers.35male rats weighing between 130g – 340g were grouped into five group of 7 each. Group 1 is the control, group 2(ethanol only), group 3 (omega 3 fatty acid only), group 4 is pre treated with Omega 3 fatty acid for 3 weeks and later induced with ethanol for 7 weeks while group 5 is induced with ethanol for 7 weeks and later post treated with Omega 3 fatty acid for 3 weeks. The study was for 10 weeks, after which the rats were sacrificed and the triglyceride and cholesterol concentration was determined. Result showed that ethanol had significant effect on the lipid concentration while omega -3 fatty acid has no protective effect on the effect of ethanol on lipid concentration in the testes of rats but has ameliorative effect.
Keywords: Testicular cholesterol, Triacylglycerol, Omega -3 fatty acid and Ethanol
Cite this paper: Adetona Mutiat Olutope, Solanke Adebayo Solomon, Amusan Kabiru Ayantayo, Ameliorative and Protective Effect of Omega 3 – Fatty Acid on Testicular Lipid Concentration in Ethanol- Induced Wistar Rats, American Journal of Biochemistry, Vol. 4 No. 2, 2014, pp. 25-28. doi: 10.5923/j.ajb.20140402.02.
Article Outline
1. Introduction
- Alcohol abuse in men causes impaired testosterone production; shrinkage of the testes (Lieber, 1995) reduced sperm counts, abnormal sperm shapes and altered sperm motility (Armstrong, et. al., 1999). The testis is sensitive to a variety of stressors and exposure to agents that induce germ cell apoptosis (O’Bryan et. al., 2000). This organ has fairly high concentrations of antioxidant (Rao and Shaha 2000). These facts indicate that the defense against oxidative stress plays critical roles in the maintenance of spermatogenesis. Excessive use of alcoholic beverages results in a variety of medical and psycho-sociological disturbances that identify alcoholism as one of modern society’s major problems (Cebral et al., 1997). Some studies have however reported that ethanol delays certain aspects of sexual maturation (Cebral et al., 1997). Reports have demonstrated that excessive ethanol consumption can produce oxidative stress and induce testicular damage (Maneesh et. al., 2005). Moreover, an increased intake of ethanol is known to increase the levels of lipids, leading to hyperlipidemia (Balasubramaiyan et. al., 2003). Studies have indicated that alcohol abuse in men can cause impaired testosterone production and testicular atrophy (Adler, 1992). Those changes can result in impotence, infertility, and reduced male secondary sexual characteristics. Testicular atrophy results primarily from the loss of sperm cells and decreased diameter of the seminiferous tubules (Van Thiel et. al., 1974). Cholesterol is an essential part of every cell in the body. It is necessary for new cells to form and for older cells to repair themselves after injury. Cholesterol is also used by the adrenal glands to form hormones such as cortisol, by the testicles to form testosterone, and by the ovaries to form estrogen and progesterone Spermatogenic cells occupy 95% of testicular volume. Therefore, failure of spermatogenesis may be characterized by testicular atrophy associated with oligospermia or azoospermia (Wright et. al., 1991). Omega-3 fatty acids play an important role to regulate genes that are critical for controlling lipid homeostasis (Sampath and Ntambi, 2005). In addition, omega-3 fatty acids could promote b-oxidation simultaneously in mitochondria and/or peroxisomes, possibly through the activation of peroxisome PPAR-a, leading to the reduction of fatty acids substrate for triglyceride synthesis and reduce total cholesterol (Oi K et. al., 2004). In view of this, the present study was conducted to investigate the ameliorative and protective effect of omega 3 –fatty acid on testicular lipid concentration in ethanol- induced wistar rats.
2. Materials and Methods
- The LD50 of ethanol extract is 5000 mg/kg in acute oral toxicity testing (Rathi et al., 2006). 25% ethanol (5g /kg body weight), Omega -3 fatty acid (2g/kg body weight) was prepared as concentration adjusted to mg/kg body weight of animals in the groups. Other reagents were of analytical grade and of the purest quality available.
2.1. Experimental Design and Administration of Drugs
2.2. Animals
- 35 male rats weighing between 130g – 340g were used.
2.3. Experimental Design
- Before administration, the rats were weighed and their weights ranged between 130g and 340g. They were then randomly divided into 5 groups consisting of seven rats each. Group 1 served as control and the other 4 groups served as experimental groups. The rats were divided into five groups with seven rats in each group.
 Group 1 is the control, group 2 was administered ethanol only, group 3 was administered omega 3 fatty acid only, group 4 was pre treated with Omega 3 fatty acid for 3 weeks without ethanol after which it was induced with ethanol for 7 weeks without omega -3 fatty acid. While group 5 was induced with ethanol for 7 weeks only without omega -3 fatty acid and later post treated with Omega 3 fatty acid for 3 weeks without ethanol.
Group 1 is the control, group 2 was administered ethanol only, group 3 was administered omega 3 fatty acid only, group 4 was pre treated with Omega 3 fatty acid for 3 weeks without ethanol after which it was induced with ethanol for 7 weeks without omega -3 fatty acid. While group 5 was induced with ethanol for 7 weeks only without omega -3 fatty acid and later post treated with Omega 3 fatty acid for 3 weeks without ethanol.2.4. Tissue Preparation
- At the end of 10th week, the rats were sacrificed and the lipid content of the testis was extracted using chloroform: methanol (2: 1 v/v). The lipid content was subjected to analysis. The Biochemical Analysis Performed Included1) Determination of triacylglycerol level: the method used is based on Enzymatic hydrolysis of serum triglycerides by lipase generates free fatty acids and glycerol. The glycerol released is phosphorylated by adenosine triphosphate (ATP) forming glycerol-1-phosphate (G-1-P) and adenosine-5’- diphosphate (ADP) in the reaction catalyzed by glycerol kinase. G-1-P is then oxidized by glycerol phosphate oxidase to dihydroxyacetone phosphate (DAP) and hydrogen peroxide (H2O2). A quinoeimine dye is produced by the peroxidase catalyzed coupling of 4- aminoantipyrine (4-AAP) and sodium N-ethyl-N- (3-sulfopropyl)m-anisidine (ESPA) with H2O2, which shows an absorbance maximum at 540nm. The increase in absorbance at 540nm is directly proportional to glycerol (and triglyceride) concentration of the sample.2) Determination of cholesterol level: cholesterol level in the brain was determined according to enzymatic colorimetric method which involves Cholesterol Enzymatic Assay Kit is a simple, direct and automationcompatible method for measuring cholesterol levels. This kit uses a coupled enzymatic reaction scheme: cholesterol esters are first converted to cholesterol and fatty acids. Next, cholesterol is oxidized with O2 to form cholesten-3-one + H2O2. Lastly, the hydrogen peroxide is reacted with 4-aminoantipyrine and p-HBS to yield quinoneimine (red dye) and water. The absorption measured at 520 nm, is proportional to the concentration of cholesterol in the sample. The kit also comes with a control solution containing a cholesterol standard (200 mg/dl) which can be used to calibrate the assay.
2.5. Statistical Analysis
- Data were subjected to a one way analysis of variance and the significance of the difference between means was determined by Duncan’s multiple range test (P <0.05).
3. Results
- PROTECTIVE AND AMELIORATIVE EFFECT OF OMEGA 3 –FATTY ACID ON TESTICULAR LIPID CONCENTRATION IN ETHANOL- INDUCED WISTAR RATS.
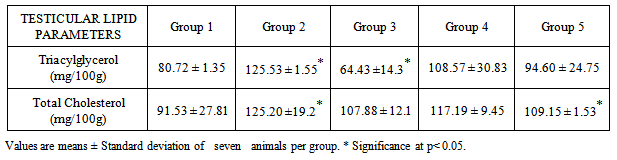 Testicular lipid concentrations of rats in the various groups were analysed and presented in the table above. It was observed that there was an increase in the triacylglycerol concentrations of groups administered ethanol compared to the control group. Group 4 which was administered ethanol (7 weeks) and omega- 3 – fatty acids (3 weeks as pre –treatment) has the highest triacylglycerol concentration. Comparing to the post-treated group, the post-treated has the reduced triacylglycerol. There is a significant difference in the level of triglyceride concentration but omega – 3 fatty acid has ameliorative effect on the triglyceride level compared to group 5 and group 2.Moreover, the cholesterol concentrations increased in group 2 and group 3 but decreased in group 5 compared to group 4 .The pre- treated group has a decrease in cholesterol concentration compared to the group 2 but not as low as that of post-treated group. There exist a significant difference in the cholesterol concentration of alcohol administered rats. The protective effects of omega -3 fatty acids is not significantly in the pre- treated group compared to the post treated group.
Testicular lipid concentrations of rats in the various groups were analysed and presented in the table above. It was observed that there was an increase in the triacylglycerol concentrations of groups administered ethanol compared to the control group. Group 4 which was administered ethanol (7 weeks) and omega- 3 – fatty acids (3 weeks as pre –treatment) has the highest triacylglycerol concentration. Comparing to the post-treated group, the post-treated has the reduced triacylglycerol. There is a significant difference in the level of triglyceride concentration but omega – 3 fatty acid has ameliorative effect on the triglyceride level compared to group 5 and group 2.Moreover, the cholesterol concentrations increased in group 2 and group 3 but decreased in group 5 compared to group 4 .The pre- treated group has a decrease in cholesterol concentration compared to the group 2 but not as low as that of post-treated group. There exist a significant difference in the cholesterol concentration of alcohol administered rats. The protective effects of omega -3 fatty acids is not significantly in the pre- treated group compared to the post treated group.4. Discussion
- There is a known association between excessive alcohol use and increase in lipid concentrations. Most studies on omega-3 fatty acids have shown that it is relevant to changes in concentrations of triacylglycerol (Hannuksela et. al., 2002). The post - treated group is preferred to the pre - treatment. Ethanol has been reported as a powerful indicator of hyperlipidemia in both animals and humans (Avogaro and Caffolatu, 1975). The most common lipid abnormalities during chronic alcohol consumption are known to produce hypercholesterolemia and hypertriglyceridemia (Baraona et. al., 1983). From the present study, ethanol-only treated groups showed significant increase in the ratio of total cholesterol and triglceride even though both total cholesterol and triglceride levels were not significantly different from omega 3 fatty acid pre-treated group (group 4) compared to the post treated group (group 5). There is sufficient evidence that ethanol can affect cholesterol synthesis and/or transport (Guizzetti and Costa, 2005).Specifically, Ashakumari et. al., (1993) have stated that the increased cholesterol during alcohol ingestion is attributed to the increased aˆ -hydroxyl methyl glutaryl CoA (HMG CoA) reductase activity, which is the rate limiting step in cholesterol biosynthesis. Since high cholesterol enhances synthesis of testosterone hormone. Numerous studies has shown that fish oil supplementation lowers the concentration of triglyceride but has little effect on concentrations of cholesterol. (Sheehan, 1997). The ameliorative effect of omega 3 fatty acid lipid concentration is significant but shows no protective effect. Some studies have revealed that free radical or ROS generation and lipid peroxidation might be an important mechanism in the toxicity of ethanol in the testes (Peltola et. al., 1996). These free radicals affects sperm motility by diffusing across the membrane into cells and inhibiting the activity of some vital enzymes such as glucose-6-phosphate dehydrogenase (6GPD) (Aitken et. al., 1997). This could cause a low sperm count thereby resulting into sterility. Nutritional supplementation with omega 3 fatty acids has been shown to ameliorate the effects of cardiovascular, neurological, autoimmune and inflammatory diseases (Rukkumani et. al., 2004). Clinical intervention studies and animal experiments showed that omega 3 fatty acids has anti-inflammatory properties (Maneesh et. al., 2005), which are based on their ability to antagonize the activity of arachidonic acid, thereby reducing the production of inflammatory and chemotactic derivatives and suppressing cell-mediated immune responses. Furthermore, omega 3 fatty acids suppress leukocyte activity and cell proliferation (Taati et. al., 2011).
5. Conclusions
- The major role of the testes is spermatogenesis, production of androgens and primarily testoterone. From this study, it was observed that increase in cholesterol and phospholipid concentration enhances high rate of spermatogenesis. The ameliorative effect of omega -3 fatty acid is ignificant on theffect of ethanol on lipid concentration of the testes.
6. Recommendations
- Alcoholist are advised to take omega -3 fatty acids along side with alcohol ingestion. Further studies on ameliorative effects of omega -3 fatty acid should be done.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML