-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(2): 15-24
doi:10.5923/j.ajb.20140402.01
99mTc-Labeled Ceftazidime and Biological Evaluation in Experimental Animals for Detection of Bacterial Infection
Safaa Bekheet1, M. El Tawoosy2, A. A. Massoud1, I. H. Borei3, H. M. Ghanem3, M. A. Motaleb2
1Physics Department (Cyclotron), Nuclear Research Center, Atomic Energy Authority
2Hot Laboratories Center, Atomic Energy Authority
3Faculty of Sciences, Ain Shams University
Correspondence to: Safaa Bekheet, Physics Department (Cyclotron), Nuclear Research Center, Atomic Energy Authority.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Nuclear medicine is a powerful diagnostic technique able to detect inflammatory foci in human disease by using radiolabeled antimicrobial agents. Our goal was labeling of ceftazidime with technetiun-99m using stannous chloride dehydrate as a reducing agent. Under optimum conditions, the labeling yield of 99mTc-ceftazidime complex, was 86.5 ± 1.3%, the complex was stable for 2 hours after labeling. Biodistribution studies in mice were carried out in experimentally induced infection in the left thigh using staphylococcusaureus. The ratio of bacterial infected thigh/contralateral thigh was then evaluated. The abscess-to-muscle ratio for 99mTc-ceftazidime was 5.06±0.8% while that for commercially available 99mTc-Ciprofloxacin was 3.8±0.5 % under the same experimental conditions, indicated that 99mTc-ceftazidime could be used for infection imaging.
Keywords: Ceftazidime, Technetium-99m, Labeling, Inflammation
Cite this paper: Safaa Bekheet, M. El Tawoosy, A. A. Massoud, I. H. Borei, H. M. Ghanem, M. A. Motaleb, 99mTc-Labeled Ceftazidime and Biological Evaluation in Experimental Animals for Detection of Bacterial Infection, American Journal of Biochemistry, Vol. 4 No. 2, 2014, pp. 15-24. doi: 10.5923/j.ajb.20140402.01.
Article Outline
1. Introduction
- Radiopharmaceuticals play a major role in providing the best possible solutions for inflammation diagnosis and treatment. In the last few decades, a large number of radiopharmaceuticals are developed for imaging of infection and inflammation. Radiolabeled compounds are injected intravenously and accumulate in the inflammatory lesion due to locally changed physiological condition after administration, the excretion occurs by glomerular filtration and tubular secretion within 24 hour being almost totally eliminated [1]. The conventional imaging techniques such as radiology, computerized tomography (CT) and nuclear magnetic resonance (NMR) are not capable of differentiating between inflammatory and infectious process. In addition, these techniques are based on important anatomic alterations and the possibility of a precocious diagnosis is limited. Contrary to conventional imaging techniques, imaging scintilography is based on physiological and biochemical alteration resulting from inflammatory and infectious process, and then further progress has been made on the use of labeled antimicrobial agents as selective markers for diagnosis of bacterial and fungal infections [2].The theoretical advantage of using antimicrobial agents as the localizing agent for infective foci is the selective toxicity of the compound for microbial rather than human targets. Such agents should therefore be able to distinguish between inflammations due to infection with microbial pathogens and inflammation due to injury or immune activity i.e. autoimmune disease where microbes are not involved. The antimicrobial agents have the potential to influence clinical decisions in the management of complicated conditions such as fever of unknown origin or occult infection [3]. The radiopharmaceuticals used for scintilographic detection include 67Ga-citrate, 99mTc or 111In-labeled leukocytes, 99mTc or 111In-labeled human polyclonal immunoglobin, 99mTc labeled antibiotic. There are several reasons why imaging of infection and inflammation becomes increasingly important in the next decade. The population is ageing; the application of implants and transplants is increasing. The number of immune compromised patients is growing, mainly because of frequent use of chemotherapeutic agents leading to neutropenia. Furthermore, the increased use of antibiotics leads to insensitivity for some of these pharmaceuticals [4, 5]. The first antibiotic was ciprofloxacin radiolabeled with 99mTc, which is supposed to bind DNA-gyrase and topoisomerase Iv of bacteria, as does unlabeled ciprofloxacin [6]. However, 99mTc-ciprofloxacin preparation has some disadvantages related to radiochemical purity (81±4%) and stability, so other antimicrobial agents such as levofloxacin, peflofloxacin, norfloxacin and cefoperazone were labeled with 99mTc to be used for imaging sites of infection and overcome the drawback of 99mTc-ciprofloxacin [7, 8].Ceftazidime (fortum) is one from the third generation cephalosporin has a wide spectrum of activity to the beta-lactamases, binds to the bacterial wall inhibits the synthesis of peptidoglycan and therefore inhibits the synthesis of bacterial wall which drives to bacterial death. Therapeutic actions: ceftazidime has a bactericidal effect; action depends on ability to reach and bind penicillin-binding proteins located in bacterial cytoplasmic membranes. Cephalosporins inhibit bacterial septum and cell wall synthesis, probably by acylation of membrane-bound transpeptidase enzymes. This prevents cross-linkage of peptidoglycan chains, which is necessary for bacterial cell wall strength and rigidity. Also, cell division and growth are inhibited, and elongation of susceptible bacteria and lysis frequently occur. Rapidly dividing bacteria are those most susceptible to the action of cephalosporins [9, 10]. The aim of this work was labeling of ceftazidime with technetiun-99m, study the labeling conditions and biological distribution of 99mTc-ceftazidime in inflammation bearing animals.
1.1. Materials
- All the chemical reagents were of analytical grade. Bidistilled water was used for solution preparation, with nitrogen purging in the case of labeling studies. Ceftazidimewas purchases from Glaxo Smith Kline, Pharmaceutical Company, Cairo., Egypt. Ceftazidime structure is shown in Figure (1). Albino Swiss mice weighing, 25-40 g were used for studying biodistribution [11].
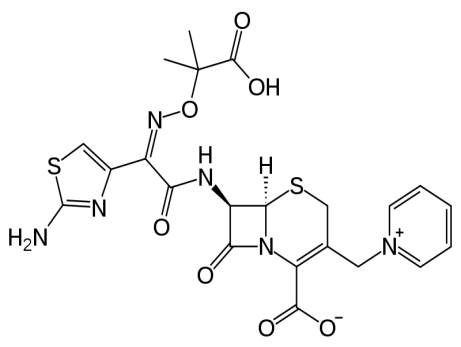 | Figure 1. Structure of Ceftazidime |
1.2. Methods
1.2.1. Labeling Procedure
- Accurately weighed 1 mg Ceftazidime was transferred to apenicillin then the vial was evacuated with nitrogen gas. Exactly 100 µl of SnCl2.2H2O stock solution (10µgSn) was added, then the volume of the reaction mixture was completed to one ml by N2-purged distilled water, added 0.5ml buffer pH 10. Finallyadded 0.5 ml of freshly eluted Na99mTcO4-(~500MBq) to the above mixture. The mixture was shaken and allows reacting at room temperature for sufficient time required to complete the reaction.
1.2.2. Radiochemical Purity
1.2.2.1. TLC Analysis
- The labeling yield and radiochemical purity were determined by layer chromatographic. The reaction product was spotted on silica gel TLC-SG strips (Sigma Chemical Company, USA) (10 x 1.5 cm2sheets). And developed in acetone and mixture, ethanol: water: ammonium hydroxide (2:5:1) as the mobile phase. After developing, the strips were dried, cut into 0.5 cm pieces and separately counted using the NaI(Tl) scintillation counter todetermine the ratio of hydrolyzed 99mTc, free 99mTcO4− and 99mTc-complex each experiment was repeated three times [12].
1.2.2.2. Electrophoresis Analysis
- It was further confirmed by electrophoresis (100 V, 30 mA, 20 W) by adding about 5 µl spot from the reaction mixture before filtration and after filtration to eliminate colloid impurities using Millipore filter of 0.22 µm size on each cellulose acetate strip, 2 cm width and 46 cm length which divided with a non-pointed pencil into fragments from zero point into 20 fragments, these strips were moistened with 0.9% N Saline solution as an electrolytes source, then placed in the chamber, after 2 hours developed strips were removed, dried and cut into one cm each fragment, then counted using gamma counter, percentage of radiochemical yield was estimated as the radioactivity of the labeled compounds to the total activity multiplied by 100 [13, 14, 15].
1.2.2.3. Stability of 99mTc-Ceftazidime
- Stability of 99mTc-Ceftazidimewas studied at different time intervals up to 24 h post labeling by TLC for determination of the percent yield of 99mTc-complex, reduced hydrolyzed technetium and free Pertechnetate.
1.2.2.4. Induction of Infectious Foci
- A single clinical isolation of Staphylococcus aureus from biological samples was used to produce focal infection. Individual colonies containing 105-106 organisms were diluted with saline in order to obtain a turbid suspension. Groups of five mice weighing 25-40 g were intramuscularly injected with 200 µl of the suspension in the left lateral thigh muscle. Then, the mice were left for 24 h to get a gross swelling in the infected thigh [16].
1.2.2.5. Induction of Non-infected Inflammation
- Sterile inflammation was induced by injecting 200 µl of turpentine oil, sterilized by autoclaving at 121ºC for 20 min, intramuscularly in the left lateral thigh muscle of the mice. Two days later, swelling appeared [17].
1.2.2.6. Induction of Heat Killed Staphylococcus Aureus Non Infected Inflammation
- Sterile inflammation was induced by injecting 200 µl of heat killed Staphylococcus aureus, sterilized by autoclaving at 121ºC for 20 min, intramuscularly in the left lateral thigh muscle of the mice. Two days later, swelling appeared [18].
1.2.2.7. Biochemical Investigations
- The blood samples were collected directly from each mice into a sterile tube, and then was centrifuged for 10 minutes to obtain the serum sample, which was stored frozen at -20ºC until analyzed. Some biochemical parameters were performed before and after inflammation induction as a markers for inflammation such as: C3, C4, LDH, CPK, ALT, AST, Albumin, TotalProtein, Urea, Creatinine [19].
1.2.2.8. Biodistribution Studies
- Biodistribution of the sterile 99mTc-ceftazidime complex was evaluated in male Albino Swiss mice weighing 25-40 g. For the quantitative determination of organ distribution, five mice were used for each experiment and 0.1 ml of about 37 MBq of 99mTc-Ceftazidime solution was injected into the tail vein of mice after 24 h of bacterial induction. The mice were anesthetized with chloroform then sacrificed. Samples of infected muscle contralateral, normal muscle, blood, bone, liver, kidney, stomach, intestine, spleen, lung, heart, and urinary bladder were weighed, and the radioactivity was measured using a gamma counter. The results were expressed as the percent uptake of injected dose per gram of tissue (ID/g) Blood, bone and muscles were assumed to be 7, 10, 40%, respectively, of total body weight. Corrections were made for background radiation and physical decay during experiment. Both target/non target thighs were dissected and counted. Target/non-target thigh radioactivity ratio was also determined [20].
1.2.3. Statistical Analysis
- Data were expressed as mean ±SD. Statistical analysis was performed using analysis of variance (ANOVA) with multiple comparison tests. A probability of less than 0.05 was considered to be significant.
2. Results and Discussion
- Radiochemical purity and stability of 99mTc-ceftazidime complex were assessed by thin layer chromatographic method. In thin layer chromatography using acetone as the solvent (about 15-30 min required for developing solvent). Free pertechnetate moves with the solvent front (Rf=1), while 99mTc-ceftazidime complex and reduced hydrolyzed technetium remained at the origin. Reduced hydrolyzed technetium was determined by using mixture ethanol: water: ammonium hydroxide mixture (2:5:1) as the mobile phase where reduced hydrolyzed technetium remains at the origin (Rf = 0) while other species migrate with the solvent front (Rf = 1). The radiochemical purity was determined by subtracting the sum of the percent of colloid and free Pertechnetate from 100%. The radiochemical yield is the mean value of three experiments [21].On the other hand, the electrophoresis purification of 99mTc-ceftazidime complex was presented in Figure (2) and showed two peaks, one at fraction No. zero, which corresponds to 99mTc- ceftazidime complex (86%) while the second peak was collected at fraction No. 8, which corresponds to free 99mTcO4- (14%), while the colloid remove by filtration using milipore filter. While Figure (3) represent the electrophoresis for free Pertechnetate only which forms one peak started from fragment No. 8 [22, 23].
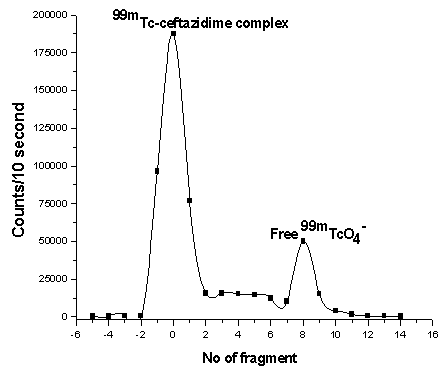 | Figure 2. Electrophoresis radiochromatogram of 99mTc-ceftazidime |
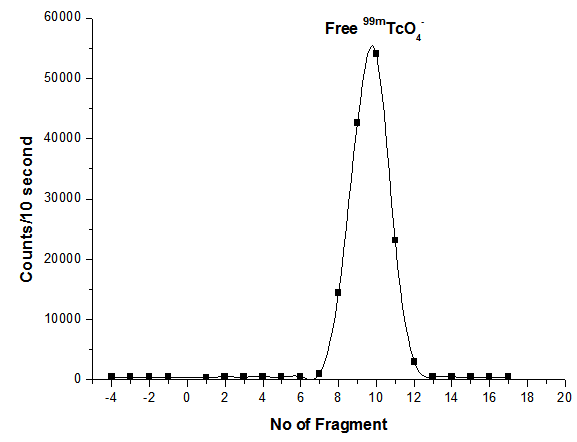 | Figure 3. Electrophoresis radiochromatogram of free 99mTcO4- |
2.1. Factors Affecting the Labeling Yield
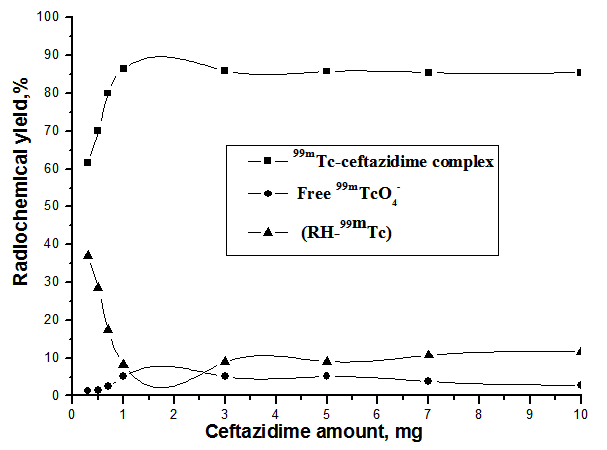
2.1.1. Effect of Substrate Amount
- Figure 4 shows that, the radiochemical yield of 99mTc- ceftazidime complex increased from 61.7% at 0.3 mg of ceftazidime to 86.5% at 1 mg ceftazidime by increasing the amount of ceftazidime. When the ceftazidime amount increased above 1mg the labeling yield slightly decreased, then above 3 mg of ceftazidime, the labeling yield remained stable. So, the optimum amount of ceftazidime was 1 mg, below This amount the yield decrease due to the fact that at low ceftazidime amount, SnCl2.2H2O is greater than that of ceftazidime, and is easily converted to colloid as the percentage of colloid was 37% using 0.3 mg ceftazidime.
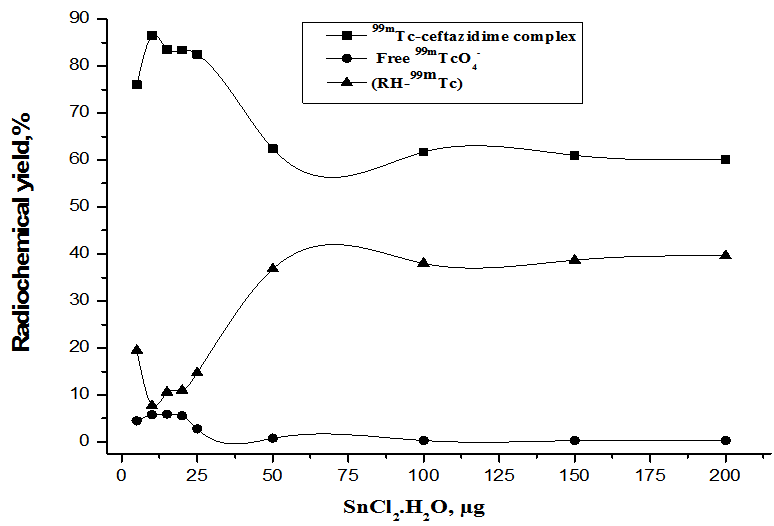
2.1.2. Effect of 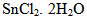 Amount
Amount
- As shown in Figure. 5, at 1 µg of SnCl2.2H2O the radiochemical yield of 99mTc-ceftazidime complex was (76%) and this low yield due to SnCl2.2H2O amount was insufficient to reduce all pertechnetate present in the reaction mixture. It was observed that the yield increased by increasing the amount of SnCl2.2H2O till reached 86.5% at 10µg SnCl2.2H2O due to sufficient amount of SnCl2.2H2O to reduce all pertechnetate present in the reaction mixture. By increasing the amount of SnCl2.2H2O the labeling yield decreased gradually till reached to (60.1%) at 200 µg SnCl2.2H2O due to colloid formation. This may be due to the fact that most of the ligand molecules were consumed in the formation of complexes, so the pertechnetate is reduced to insoluble technetium (IV) TcO2.xH2O in the absence of ligand [20] or due to the fact that the excess amount of stannous chloride leads to the formation of stannous hydroxide colloid Sn(OH) [27].
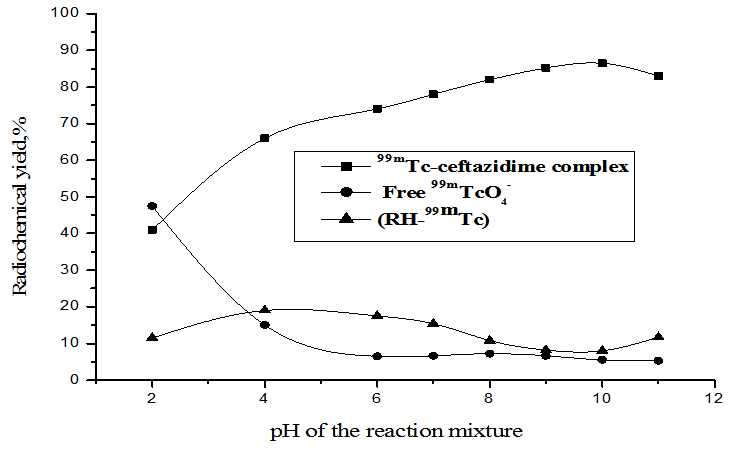
2.1.3. Effect of pH of the Reaction Mixture
- As shown in Figure. 6, at pH 2, the labeling yield of 99mTc- ceftazidime complex was 41% and the yield increased with increasing the pH of the reaction mixture to a maximum yield of 86.5% at pH 10. At pH below or above the optimum pH, the radiochemical purity is significantly decreased by forming reduced hydrolyzed technetium-99m which is the main radiochemical impurity.
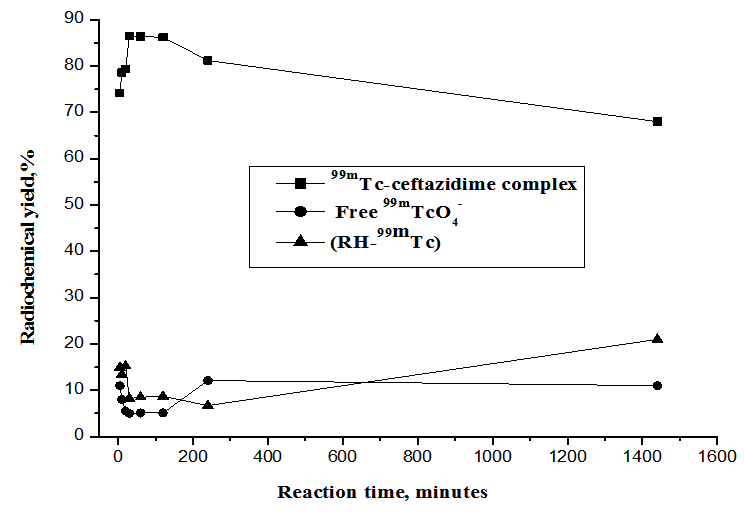
2.1.4. Effect of Reaction Time and Stability
- As shown in Figure 7, the labeling of ceftazidime with technetium-99m was done at room temperature (25ºC) and carried out at different intervals of time. The labeling reaction was complete after 30 min with a radiochemical yield of 86.5%. The formed complex was stable for a time up to 2 h, after that the yield decreased till reached 68% after 24 h from the post labeling due to colloid formation.
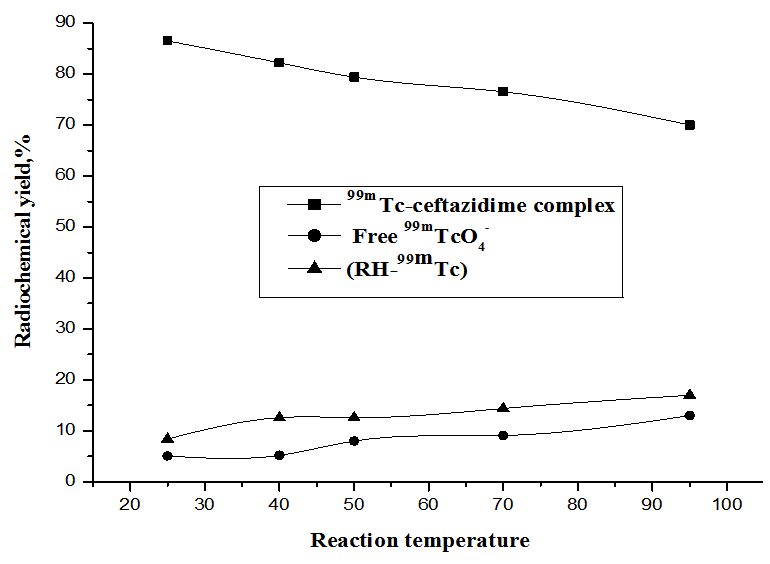
2.1.5. Effect of Reaction Temperature
- As shown in Fig. 8, the radiochemical yield of 99mTc- ceftazidime complex was maximum (86.5%)at room temperature 25ºC, then decreased gradually to reached (70%) at 95ºC.
2.2. Biodistribution in Animals
2.2.1. Biochemical Investigations
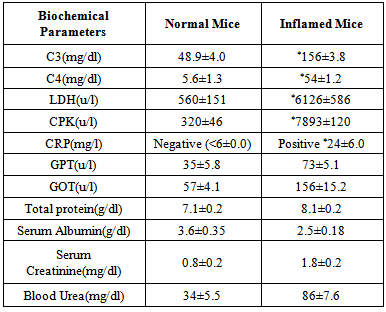
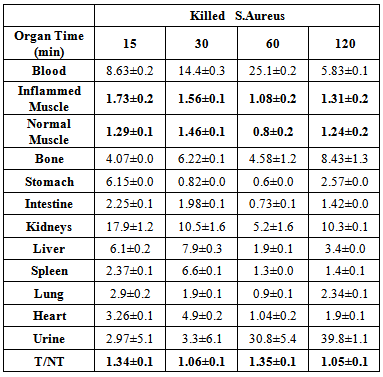
- The results in table 1 showed increasing in the biochemical parameters results after inflammation with bacteria induction in animals than before inflammation induction, liver enzymes (aspartate aminotransferase AST, alanine aminotransferase ALT), kidney function tests (serum creatinine, blood urea nitrogen and total protein) were increased in state of inflammation than in normal mice, while serum albumin was decreased in state of inflammation [23]. There were high increase in the muscle markers enzymes Lactate Dehydrogenase (LDH), Creatinine Phosphokinase (CPK) also high increase in amount of complement 3, complement 4, c-reactive protein (CRP) in state of inflammation than in normal mice, which play an important role in host defense mechanisms against infection, The human immune system is notable for its ability to combat infectious microorganism by cliciting inflammatory responses such as acute phase protein (CRP) which elevated markedly in association with infection and inflammation [28, 29].
|
2.2.2. Bidistribution
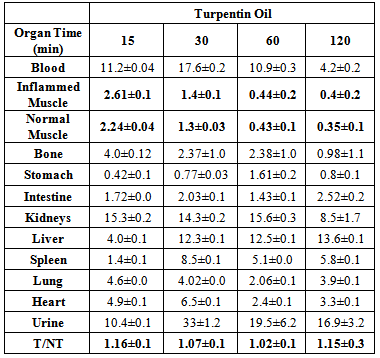
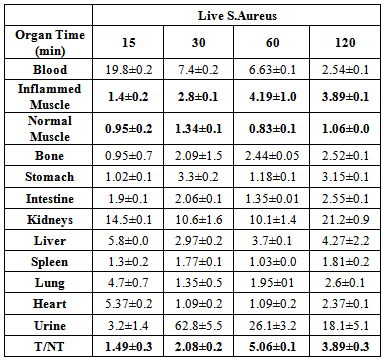
- The uptake of the 99mTc-ceftazidime complex in different organs of the animals infected with turpentine oil, s. aureus and killed s.aureus was given in table (2a,b,c), respectively. 99mTc-ceftazidime was removed from the circulation mainly through the urinary pathway after 2 h post injection of the tracer. In the turpentine oil inflamed mice, a significant large amount of 99mTc-ceftazidime activity was observed in the liver (12.3%) after 30 min post injection. After 2 h of tracer administration, the major part of activity of 99mTc-ceftazidime was found in the kidney (8.5±1.7), liver (13.6±0.1) and spleen (5.8±0.1) as shown in table 2 a. In contrast, in s.aureus inflamed mice, the liver uptake decreased than that in turpentine oil from 2.97% after 30 min till reaching 4.27% after 2 h as shown in table 2b. The uptake of 99mTc-ceftazidime was significantly low in turpentine, killed s.aureus infected group of animals (Aseptic inflammation) as compared to infected group with living bacteria (abcess). These data indicate that the distribution throughout the body and uptake in the inflamed area was observed within 60 min after intravenous injection of the tracer.
|
|
|
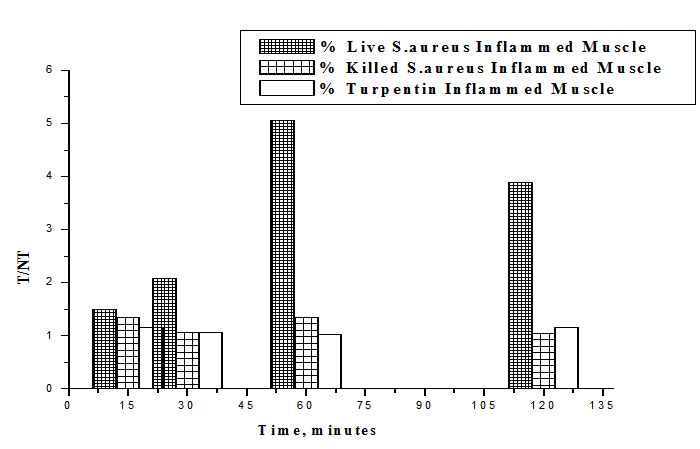 | Figure 9. T/NT of live S.aureus, Killed S.aureus and turpentine oil at 15, 30, 60, 120 min post injection 99mTc-Ceftazidim |
3. Conclusions
- 99mTc-ceftazidime was labeled easily using 1 mg Ceftazidime, 100 µl of SnCl2.2H2O (10 µg Sn) as reducing agent, 3 mCiNa99mTcO4-, at pH 10 with a high labeling yield of 86.5 ±1.3%, using simple and instantaneous method with stability (up to 2 h) post labeling. 99mTc-ceftazidime was accumulated at the site of infection withT/NT ratio=5.03 higher than that of the commercially available 99mTc-ciprofloxacin (labeling yield ~ 80%, stable up to 2 h post labeling and T/NT=3.8). So 99mTc-ceftazidime showed better yield and biodistribution for imaging of infection at early stages and distinguishing infection from sterile inflammationthan 99mTc-ciprofloxacin.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML