-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2014; 4(1): 1-5
doi:10.5923/j.ajb.20140401.01
Determination of Inhibitory and Flavour Compounds in Fermented Camels Milk (Gariss)
Abdallah A. Alyan 1, Abdel Moneim E. Sulieman 2, Abdelrahim A. Ali 3, Abdlulaziz S. Bahobail 4
1Department of Biotechnology, Faculty of Science, Taif University, Kingdom of Saudi Arabia
2Department of Biology, faculty of Science, University of Hail, Kingdom of Saudi Arabia
3Department of Chemistry, Faculty of Science, Taif University, Kingdom of Saudi Arabia
4Department of Miccrobiology, Faculty of Science, Taif University, Kingdom of Saudi Arabia
Correspondence to: Abdel Moneim E. Sulieman , Department of Biology, faculty of Science, University of Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
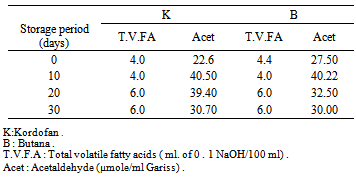
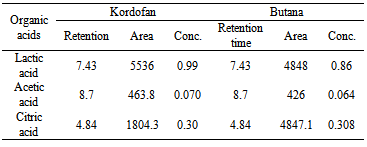
In the present study, some of the inhibitory compounds such as lactic acid, acetic acid and citric acid were determined in Gariss samples obtained from two sources, Kordofan (Western Sudan) and Botana (Central Sudan). The mean values of these acids contents were 0.99, 0.070 and 0.30 (mg/100g) in Kordofan Gariss, and 0.86, 0.064 and 0.308 (mg/100g) in Botana Gariss respectively. The flavour compounds such as acetaldehyde and volatile fatty acids found in Gariss were also determined, however, the mean values of the acetaldehyde contents at 0, 10th, 20th and 30th day of storage were 22.60, 40.50, 39.40 and 30.70 (µmol/ml) in Kordofan Gariss, and 22.50, 40.22, 32.50, 30(µmol/ml) in Botana Gariss, respectively. However, the mean values of total volatile fatty acid contents at 0, 10th, 20th and 30th day of storage were 4.0,4.0,6.0,6.0, of 0.1 ml NaOH/10ml in Kordofan Gariss 4.4,4.0,6.0,6.0 and in Butana Gariss s, respectively.
Keywords: Acetaldehyde, Lactic acid, Citric acid, Volatile fatty acids
Cite this paper: Abdallah A. Alyan , Abdel Moneim E. Sulieman , Abdelrahim A. Ali , Abdlulaziz S. Bahobail , Determination of Inhibitory and Flavour Compounds in Fermented Camels Milk (Gariss), American Journal of Biochemistry, Vol. 4 No. 1, 2014, pp. 1-5. doi: 10.5923/j.ajb.20140401.01.
Article Outline
1. Introduction
- Fermentations transform the original food by producing acids, alcohols and volatile Compounds that add flavor and aroma. Some of these chemicals are antimicrobials. They inhibit the growth of undesirable pathogens and Spoilage microbes. Thus fermentation preserves food. Generally, fermentation is a self-limiting process. The accumulating acids and alcohols eventually Kill even The fermenting microorganisms themselves.Microorganisms of lactic acid starter cultures used for the conversion and preservation of milk by-products are unique bio-converters of energy. When they are used appropriately, these cultures elaborate specific metabolites during fermentation these metabolites, in conjunction with partial hydrolysis of milk constituents (proteins, fat and lactose) contribute to better digestibility of the fermented food and their nutritional and therapeutic qualities also are enhanced.The camel population in Sudan was estimated at 3.724 million head according to the Ministry of Animal Resource and Fisheries[1]. In Sudan, camel’s milk and the fermented camel’s milk (Gariss) are widely consumed by the rural people and pastoralist communities inhabiting the arid and semi-arid regions of the country.Flavour is a crucial characteristic of foods as the sensory characteristics play an important role in product acceptance by consumers. In fermented dairy products, flavor perception is strongly based on the volatile components[2]. The most prominent ones are mainly lactic acid which imparts an acidic and refreshing taste, and a mixture of various carbonyl compounds like acetaldehyde, ethanol, acetone, diacetyl and 2-butanone. However, among them, acetaldehyde is considered as the major flavor compound for the typical yogurt aroma reported by several researchers [3][4]. Gariss which is prepared from camel’s milk, is prepared made by semi-continuous or fed-batch fermentation process in large skin bags or siin, which contains a large quantity of previously soured product[5]. The objective of the present study was to determine the contents of the inhibitory compounds as well as flavour components of fermented camels milk product (Gariss).
2. Materials and Methods
- Traditional Gariss samples were obtained different locations in Kordofan “Western Sudan” and Butana “Eastern Sudan”. Each sample was transferred to 250 ml autoclavable plastic container. The samples were Kept at 4°C and transported to the Food Technology Research institute-Egypt. Analyses were carried out at 0, 10th, 20th, and 30th day of storage at 4°C.
2.1. Determination of Organic Acids by HPLC Methods
- Organic acids contents of Gariss product were determined using high performance liquid chromatography (HPLC).
2.2. Sample Preparation
- Samples were prepared by adding 25ml of a 0.0065M sulphuric acid solution to 5g Gariss sample , then stirred for two hours. The supernatant was extracted by filtering through a 0.20 mm disposable syringe filter, then the sample were added to the tube and transferred to injection vials. The standard organic acid was treated same as the sample. The concentration was then calculated as follows:
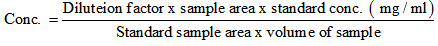
2.3. Determination of Acetaldehyde Content
- The volatile compounds in the samples were determined by using static headspace gas chromatographic method. During storage, ten grams of sample transferred in 20 mL headspace vial (Agilent, USA), which was sealed with PTFE/BYTL headspace septa (Agilent, Germany) and aluminum cap (Agilent, USA). Samples were kept at –20℃ until analyses. Prior to analysis, frozen samples of (10 g) were thawed at 4 oC overnight. The vials with samples were held at 60°C for 1hour, then at 75°C for 10 min. and stirred 5 times, and subsequently kept for 5 min. Headspace (250 μL) was injected with gas-tight syringe onto the GC column using split mode (10:1). The temperature of syringe was kept at 80°C. All glass materials (HS vials, volumetric flasks) were sterilized before use. Double distilled water to be used for preparation of standard solutions was boiled for 20 minutes to remove residual volatiles and was subsequently stored in a stopped glass container. All chemicals were analytical grade supplied by Merck Ltd. (Merck House Poole Dorset England BH15 ITD, UK). The volatile compounds were separated on a HP INNOWAX capillary column (30 m x 0.32 mm id x 0.25 μm film thickness) under the following conditions: injector temperature 200°C; carrier gas helium at a flow rate of 1.4 mL/min; oven temperature program initially held at 50°C for 6 minutes and then programmed from 50°C to 180°C at 8 oC/min and held at 180°C for 5 minutes. The GC column was connected to the Agilent 5973N model mass selective (MS) detector (Agilent, USA) which was operating in the scan mode within a mass range of 33 to 330 m/z at 1 scan /s. The interface line to MS was set at 250°C. The MS was operated in an electron impact mode at electron energy of 70 eV. The compounds were identified by a computer-matching of their mass spectral data with those of known compounds from the Nist 02.L. Mass Spectral Database. Based on the peak resolution, their areas were estimated - from the integrations performed on selected ions. The resulting peak areas were expressed in the arbitrary area units. Quantification of constituents was calculated by external standard technique. For this purpose, authentic standards of acetaldehyde, accurately weighed and dissolved in 10 mL of double distilled water. Five sets of concentrations prepared in the range of 1 to 30 ppm. Results achieved from the collections of standards were used to calculate a mean peak area for each standard compound. All collections were made in triplicate and consequently the amounts of each compound in sample were calculated over known amount of standard and its peak area
2.4. Total Volatile Fatty Acids
- The total volatile fatty acids were determined by gas – chromatographic method (chromatograph-Vega sene 6000 Carlo Erba). Condition of this analysis were: Capillary column type, Nukol, length 15 m, internal diameter 0.53 mm, injector temperature 200°C, detector flame ionization temperature 220°C, air pressure 120 kPa, H, pressure 70kPa, Temperature program (isotherm) °C duration 6 minutes, Carrier pressure 30kPa.
3. Results and Discussion
3.1. Acetaldehyde Contents
- Acetaldehyde is a sweet-smelling compound, produced in the normal breakdown of carbohydrates. It is an aroma and flavour compound in dairy products which is produced by lactic acid bacteria, and it is also described as having an antimicrobial activity against Bacillus spp.[6] and is more active against Gram negative bacteria and molds.Table (1) shows the mean values of the acetaldehyde contents, which were 22.60,40.50, 39.40 and 30.70 (µmol/ml) in Kordofan Gariss, 22.50, 40.22, 32.50, 30 (µmol/ml) for Botana Gariss at at 0, 10th, 20th and 30th day of storage, respectively.
|
3.2. Total Volatile Fatty Acid
- Table (1) show mean values of total volatile fatty acid contents (T.V.F.A) which were 4.0 ,4.0 ,6.0, 6.0 of 0.1 ml NaOH/10ml in Kordofan Gariss while Butana Gariss contained 4.4, 4.0, 6.0, 6.0 at 0, 10th, 20th and 30th day of storage, respectively .The values of total volatile fatty acids of Gariss samples increased slightly during storage. These results are in agreement With those reported by other investigators[10] The values of T. V. F. A ranged between (4-6.3ml of 0.1 NaOH/10ml ), this range is lower than that reported by Mehanna et al (1988) for cows yoghurt which were (8-12ml of 0.1ml/NaOH/10ml) this could be due to low content of short chain fatty acids in camels milk fat than of cows[11].
3.3. Organic Acids
- During fermentation of milk, the concentration of some organic acids such as lactic, propionic and acetic increases, while the concentration of others such as orotic and citric decreases[12][13] depending on the microorganisms involved. In addition, benzoic, sorbic and nucleic acids also takes place in the fermentation process of milk in lower [14][15]. Nevertheless their role as natural preservatives is very important for the increasing quality of the fermented milk products, especially for extending their shelf life.The antimicrobial activity of lactic acid bacteria isolated from milk products is used in biological preservation[16]. The organic acids are inhibitory compounds produced by lactic acid bacteria. The antimicrobial activity is more powerful at low PH. Acetic acid, lactic acid and citric acid are strong inhibitors. Mixture of lactic acid and acetic acid reduces the growth of Salmonella typhimurium[17]. Several short-chain organic acids are found as products of milk fermentation. They are measured to assess the level of fermentation or added as antioxidants[18]. Japers[19] studied the effect of lactic acid, acetic acid and HCL against E. coli as shown in Fig. (1) Table (2) shows the concentration of lactic acid, acetic acid and citric acid in Kordofan and Butana Gariss expressed as mg/100g. - lactic acid is the main acid present in all tested samples of Gariss. - its concentration could be attributed to the fermentation. - the concentrations of citric acid in Gariss were too low to detect. - Kordofan Gariss had the highest concentration of lactic acid and Buttana.
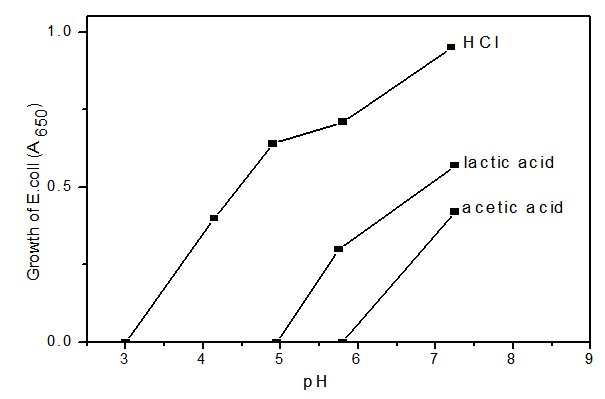 | Figure 1. Effect of hydrochloric acid,lactic acid, and acetic acid On rate of inhibition of E.coli |
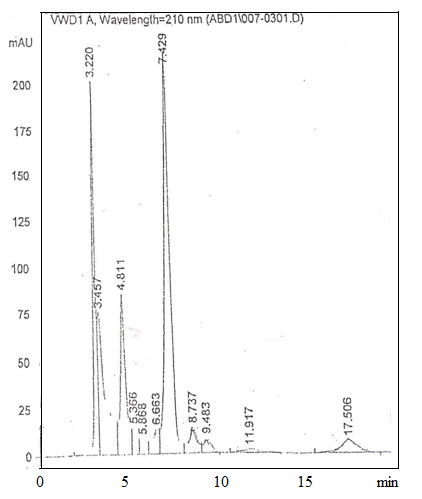 | Figure 2. Concentration of lactic acid, acetic acid and citric acid in Kordoean Gariss using (HPLC) |
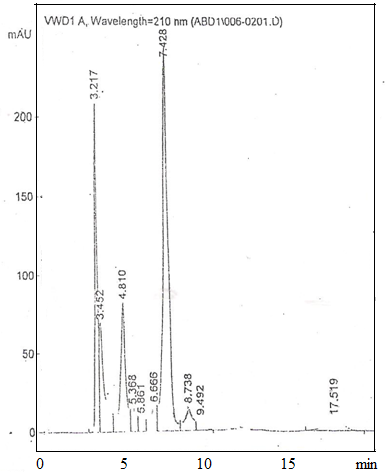 | Figure 3. Concentration of lactic acid, acetic acid and citric acid in Butana Gariss using (HPLC) |
|
4. Conclusions
- The inhibitory effect of fermented milk products could be attributed to the presence of organic acids, so, presence lactic acid, acetic acid and citric acid could contribute to the inhibitory action of Gariss. On the other, the pleasant flavour of Gariss could contribute to the as acetaldehyde and volatile fatty acid which were present in product.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML