-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(4): 97-106
doi:10.5923/j.ajb.20130304.03
Expression of Micro RNA192 in Type 2 Diabetes Mellitus Relation to Glycemic Control, Metabolic Abnormalities, Renal and Ocular Complications
Hamdia Ezzat 1, Abdelwahab M. Lotfy 2, Fatma A. Attia 3, Gihan A. Mohamed 4, Hend M. Tawfeek 3, Amany M. Abdallah 3, Asmaa I. Arafa 5
1Departments of Clinical pathology, Faculty of Medicine for Girls. Al-Azhar University, Egypt
2Department of Internal Medicine, Faculty of Medicine for boys. Al-Azhar University, Egypt
3Department of Internal Medicine, Faculty of Medicine for Girls. Al-Azhar University, Egypt
4Department of Ophthalmology, Faculty of Medicine for Girls. Al-Azhar University, Egypt
5Department of Endocrinology, Faculty of Medicine for Girls. Al-Azhar University, Egypt
Correspondence to: Hamdia Ezzat , Departments of Clinical pathology, Faculty of Medicine for Girls. Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Background: MicroRNAs are a class of naturally occurring small noncoding RNAs that regulate gene expression, cell growth, differentiation and apoptosis by targeting mRNAs for translational repression or cleavage. miRNAs associated with various pathological conditions including DM. Microvascular disease is the most frequent complication of diabetes. It is responsible for diabetic retinopathy nephropathy, and neuropathy. Purpose: This work was carried out to evaluate the expression level of miRNA 192 in type 2 diabetic patients and to assess the relation of miRNA 192 expression to glycemic control, metabolic abnormalities, renal and ocular complications aiming at early diagnosis and prevention of these complications. Patients and Methods: This study was conducted on 36 type 2 diabetic patients subdivided into 2 main groups according to onset of DM. 16 newly diagnosed cases and 20 patients with long standing DM as compared to 14 (age and sex matched) healthy persons. Patients with long standing DM further subdivided into diabetic nephropathy and patients with diabetic retinopathy. Patients were subjected to: detailed history taking and clinical examination, and laboratory investigation. The expression levels of miRNA192 in whole blood using (RT-PCR) were determined. Results: Mean expression level of miRNA 192 was significantly higher in diabetics compared to healthy subjects. This increase is more obvious for long standing diabetics than newly diagnosed diabetics. Similar findings were also noted for patients with diabetic retinopathy and nephropathy. Patients with diabetic nephropathy showed higher expression level of miRNA 192 as compared to diabetic patients with retinopathy. There was association between miRNA 192 and disease duration; there was significant positive correlation between miRNA 192 expression levels and blood glucose, HbA1c, creatinine and urinary albumin excretion rate in diabetic nephropathy subgroup. There was a significant positive correlation between miRNA 192 expression levels and blood glucose, HbA1c in patients with diabetic retinopathy. Conclusion: The study concluded that increase expression of miRNA 192 in type 2 DM, metabolic abnormalities, poor glycemic control and obesity, are risk factors related to prevalence of renal and ocular complications among diabetic subjects. Our results suggest that miRNA 192 may serve not only as molecular signatures in diabetic microvascular complications but also as early indicators of alterations in specific biological processes in the kidney, retina, and nervous system. We recommend further studies to evaluate the diagnostic, prognostic value of miRNA 192 and to evaluate anti-miRNA 192 as a therapeutic option in various diabetic microvascular complications.
Keywords: Diabetes Mellitus, miRNA 192, Diabetic Nephropathy, Diabetic Retinopathy
Cite this paper: Hamdia Ezzat , Abdelwahab M. Lotfy , Fatma A. Attia , Gihan A. Mohamed , Hend M. Tawfeek , Amany M. Abdallah , Asmaa I. Arafa , Expression of Micro RNA192 in Type 2 Diabetes Mellitus Relation to Glycemic Control, Metabolic Abnormalities, Renal and Ocular Complications, American Journal of Biochemistry, Vol. 3 No. 4, 2013, pp. 97-106. doi: 10.5923/j.ajb.20130304.03.
1. Introduction
- Diabetes mellitus is one of the commonnon-communicable diseases that are increasing globally. Type-2 DM is the most common type[1]. Several environmental and genetic factors had been incriminated in its pathogenesis and its complications[2]. Diabetes mellitus is associated with microvascular, macrovascular and non-vascular complications[3, 4].Diabetic nephropathy (DN) is one of the most common micro vascular complications of diabetes defined as rise in urinary albumin excretion rate often associated with an increase in blood pressure, but without evidence of other causes of renal disease[5]. Specifically, it represents a major cause of morbidity and mortality in diabetic subjects[6, 7]. Diabetic retinopathy is a major cause of new-onset blindness among diabetic adults and is characterized by increased vascular permeability, tissue ischemia, and neo-vascularization[8]. Early changes include micro aneurysms, hemorrhages, hard exudates, cotton wool spots, intraretinal microvascular abnormalities, and venous beading which characterize nonproliferative diabetic retinopathy (NPDR). The more severe state of proliferative diabetic retinopathy (PDR) is marked by the formation of abnormal fragile new blood vessels that are prone to hemorrhage. Finally, visual impairment results from secondary pre-retinal or vitreous hemorrhage and diabetic maculopathy[9].In recent years microRNAs (miRNAs), a family of short (average of 20~25 nucleotide long), naturally occurring, small antisense non-coding RNAs have emerged as important post-transcriptional regulators of gene expression [10]. There may be thousands of miRNA genes in the human genome, transcribed by RNA polymerase as long primary miRNA molecules, then processed in the nucleus forming pre-miRNAs[10, 11]. These pre-miRNAs get transported from the nucleus to the cytoplasm for further processing[12, 13]. It has been shown that miRNAs can affect the stability of messenger RNA (mRNA) and in some cases influence protein synthesis through partial sequence complementation with their interacting mRNA targets[14]. miRNAs are predicted to control the activity of more than 60% of all protein-coding genes[15]. It has been estimated that miRNAs regulate up to 30% of human genes[16].Like mRNAs, some miRNAs also show restricted tissue distribution; for example, miR-122 is highly enriched in liver, whereas miR-124 is preferentially expressed in neurological tissues[17]. miRNAs may induce deregulation of specific mRNAs. These factors may affect human immune response and then result in many pathogenic disorders. It has been shown that changes in the spectrum of cellular miRNAs correlate with various physiopathological conditions, including differentiation, inflammation, diabetes, and several types of cancers[18, 19].miRNA -192 is thought to be positive regulators of p53, a human tumor suppressor[20]. They are also over expressed in gastric cancer, and could potentially be used as biomarkers or therapeutic targets[21] It has also been suggested that mir-192 could be used as a biomarker for drug-induced liver damage[22].Among the miRNAs highly expressed in the kidney, several key miRNAs (miR-192, miR-200b, miR-200c, miR-216a and miR-217) were found to be higher in renal glomeruli of mouse models of diabetes[type 1(streptozotocin (STZ)-induced) and type2 (db/db)] compared to the corresponding controls[23,24] Some or all of these miRNAs were also increased by TGF-β or high glucose (HG) in mouse mesangial cells (MMC) and in human MC[24]. miR-192 targets Zeb1 and Zeb2 which are now widely recognized as general E-box repressors[25], and increases Collagen type I alpha2 (Col1a2) gene expression in MMC, demonstrating that increased miR-192 in diabetic conditions induces fibrosis by inhibiting E-box repressors[23]. Krupa et al., 2010 reported decreased miRNA -192 was noted in biopsy specimens of patients with advanced diabetic nephropathy[26]. Only two published studies have specifically looked at the role of miRNAs in diabetic retinopathy (DR)[27, 28].Aim of the study: This work was carried out to evaluate the expression level of miRNA 192 in type 2 diabetic patients and to assess the relation of miRNA 192 expression to; glycaemic control, metabolic abnormalities, renal and ocular complications aiming to early diagnosis and prevention of these complications.
2. Subjects and Methods
- The present study included 36 type 2 diabetic patients randomly selected from the regular attendants of the Diabetes Specialized Clinic, Internal medicine Clinic and from in-patients of endocrinology and Internal medicine departments at El Zahraa University Hospital over a 6 month period (from January to June 2013), as well as 14 age and sex-matched healthy persons.Patient inclusion criteria: fasting plasma glucose ≥ 126 mg/dl, Haemoglobin A1c ≥ 6.5% and/or treatment for diabetes included diet, oral antidiabetic drugs and/or insulin to achieve glycemic controlPatient exclusion criteria: Patients with a history of liver cirrhosis, malignancy, infection, inflammation, bronchial asthma, heart failure, were excluded from the study On clinical examination diabetic patients which have combined renal and ocular complications were excluded from the study as well as diabetic patients with macrovascular complication were also excluded from the study.The diabetic patients divided into two groups:Group I (G I): comprised 16 newly diagnosed cases type 2 DM in the first two years of illness. Their age ranged from 48 to 56 years with mean age of 52.7±4.4 years (8 women and 8 men), Group II: comprised 20 patients with long standing type 2 DM (duration more than 5 years). Their age ranged from 49 to 57.5 years with mean age of 54.4±5.1 years (11 women and 9 men) compared to Group III: 14 healthy persons their age ranged from 48.5 to 55.5 years with mean age of 52 ±4.1years (8 female and 6 male). Patients with long standing type 2 DM further subdivided into two subgroups according to microvascular complications. GIIa: diabetic patients with nephropathy N=11 and GIIb:diabetic patients with retinopathy N=9. This study was performed after obtaining informed consent from all participating subjects.All Patients and control were subjected to the following assessments:Thorough history taking and clinical examination including measurement of BMI, body mass index is defined as the individual's body weight divided by the square of his or her height in meter (kg/m²). Assessment of the patients for the presence of diabetic microvascular complications was done as the following; diabetic peripheral neuropathies were diagnosed by full neurological examination. Assessment for diabetic retinopathy was done by best corrected visual acuity, slit-lamp biomicroscopy and indirect ophthalmoscopy, examination of the fundus of the eye bilaterally in fully dilated pupils using the ophthamoscope. Slit lamp examination, fundus fluorescein angiography, optical coherence tomography imaging of the posterior segment of the eye. Diagnosis was made when we found retinal hemorrhage, exudates, retinal micro aneurysms, new vessels and/or vitreous hemorrhage. The degree of retinopathy for each patient was determined. A useful clinical classification according to the types of lesions detected on fundoscopy was as follows:**Mild non-proliferative diabetic retinopathy: Micro aneurysms, Dot blot hemorrhages and hard (intra-retinal) exudates.**Moderate-to-severe non-proliferative diabetic retinopathy: The above lesions usually with exacerbation, plus: Cotton-wool spots, venous beading, loops and Intra retinal micro vascular abnormalities (IRMA) as shown in figure 1.
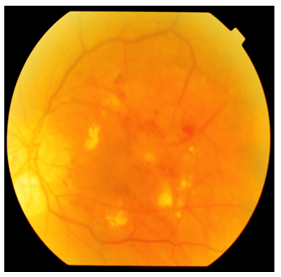 | Figure 1. Severe NPDR with clinical significant macular edema (Lt eye) |
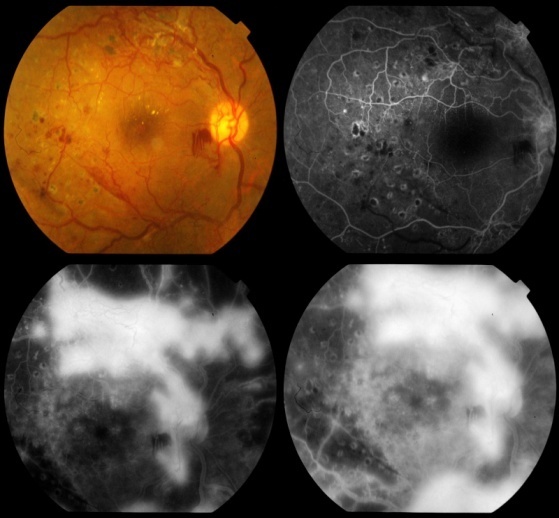 | Figure 2. Proliferative diabetic retinopathy with neovascularization |
3. Results
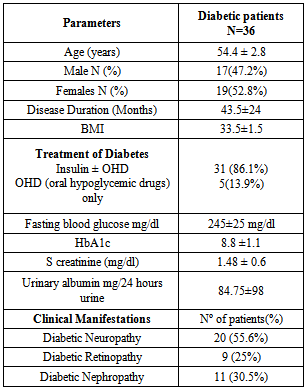
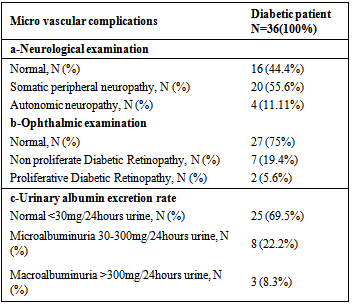
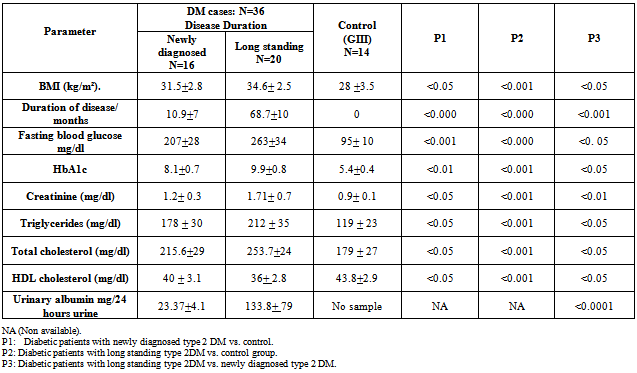
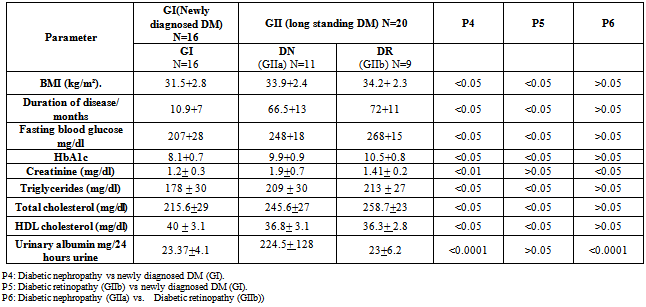
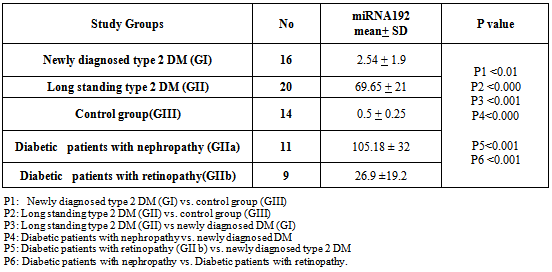
- Demographic data, clinical and laboratory findings of all 36 diabetic patients were shown in table 1&2. Clinical and laboratory findings of diabetic groups, newly diagnosed cases and long standing type 2 DM as compared to control Group (GIII) were shown in table 3.
|
|
|
|
|
|
4. Discussion
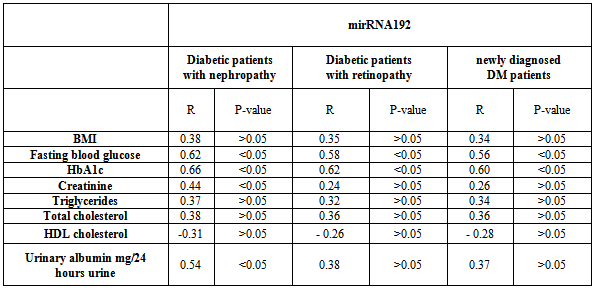
- microRNAs (miRNAs) are short non-coding RNAs regulating gene expression at the post-transcriptional level by blocking translation or promoting cleavage of their target mRNAs[31]. The miRNA molecules are synthesized in the nucleus through RNA polymerase II. They are then processed to precursor miRNAs (70–100 nucleotides, hairpin-shaped) in the nucleus by RNAse III Dorsha and DiGeorge syndrome critical region 8 (DGCR8). The miRNAs are then exported to the cytoplasm by exportin 5. Finally, an active form (20–25 nucleotides) is produced in the cytoplasm after further processing by Dicer. MiRNAs have significant effects on the regulation of gene expression. They bind to the specific mRNA targets, causing their degradation or translational repression[32]. Several investigators have used overexpression experiments to demonstrate the importance of miRNAs in diverse cellular processes. Increasing evidence shows that miRNAs play central roles in gene transcription, signal transduction and pathogenesis of human diseases[33].The effect of miRNAs is via the incomplete binding of the “seed sequence” at the 5′ end of the miRNA to the complementary target site in the 3′ untranslated region (UTR) of the messenger RNA. Recent studies have demonstrated clear links between altered miRNA expression and certain diabetes complications,[34].Hyperglycemia is responsible for the initiation and the progression of chronic diabetes complications contributing to the structural and functional changes in the retina and other organs. Despite the identification of multiple pathogenetic mechanisms in a complex problem like diabetic retinopathy (DR), limited success has been achieved in its medical treatment[27]. Diabetic nephropathy (DN) is a severe microvascular complication that can lead to end-stage renal disease. Increased expansion and accumulation of extracellular matrix (ECM) proteins such as collagen in the glomerular mesangium along with glomerular podocyte dysfunction are major features of DN. Profiling of miRNAs and study of their functions in renal glomeruli can provide critical new information to advance our knowledge of DN[35].In the present study we investigated whether miRNA 192 alterations are involved in diabetic microvascular complications and to assess the relation of miRNA 192 expression to glycemic control, metabolic abnormalities, renal and ocular complications aiming at early diagnosis and prevention of these complications.The results of the present study have shown a significantly higher mean expression level of circulating miRNA 192 from both diabetic groups than control group, also our work demonstrated a significant increase in mean expression levels of miRNA192 from long standing DM when compared with newly diagnosed DM. Our results were in agreement with Kato et al., 2007 who observed increased renal expression of miRNA -192 in streptozotocin (STZ)-induced diabetes and in the db/db mouse and demonstrated that tranforming growth factor (TGF-β1) upregulated miRNA -192 in mesangial cells (MCs) (23). miRNA 192 repressed the translation of ZEB2, a transcriptional repressor that binds to the E-box in the collagen 1a2 (col1a2) gene. They proposed that miR-192 repressed ZEB2 and resulted in increased (col1a2) expression in vitro and contributed to increased collagen deposition in vivo. These data suggest a role for miR-192 in the development of the matrix accumulation observed in DN. More recently Chung et al., 2010 revealed the regulatory mechanisms by which TGF-β1 regulates miR-192 expression via the Smad3 but not the Smad 2 signaling pathway[36].In contrast to our results Krupa et al., 2010 reported decreased miRNA -192 was noted in biopsy specimens of patients with advanced diabetic nephropathy, In that report, they concluded that miRNA -192 expression was predominantly localized to tubular epithelial cells and TGF exposure was found to decrease both miR-192 and E-cadherin mRNA levels and they concluded that a decrease in miR-192 is associated with increased renal fibrosis in vivo[26].Our work demonstrated a significantly higher mean expression level of circulating miRNA 192 from patients with diabetic nephropathy as compared to patients with diabetic retinopathy, and patient with newly diagnosed type 2DM. This can suggest a possible role of miRNA192 in pathogenesis and progression of microvascular complications in diabetic patients. Putta et al reported that TGF-β1 upregulates miRNA192 in cultured glomerular mesangial cells and in glomeruli from diabetic mice. miRNA 192 not only increases collagen expression by targeting the E-box repressors Zeb1/2 but also modulates other renal miRNAs, suggesting that it may be a therapeutic target for diabetic nephropathy They concluded that specific reduction of renal miR-192 decreases renal fibrosis and improves proteinuria[37].We also demonstrated higher mean expression levels of circulating miRNA 192 from patients with diabetic retinopathy as compared to patient with newly diagnosed DM. Only two published studies have specifically looked at the role of miRNAs in DR, McArthur et al., 2011 investigated whether miRNA alterations are involved in DR in a STZ-T1DM rat model, they studied the role of miR-200b, which targets VEGF-A, they reported that miR-200b down-regulated in the retinas of diabetic rats[27]. Kovacs et al., 2011 performed miRNA expression profiling in the retina and retinal ECs of STZ-T1DM rat and they reported that miRNAs are involved in the pathogenesis of DR through the modulation of multiple pathogenetic pathways and may be novel therapeutic targets for the treatment of DR[28].It is generally believed that the hyperglycemic environment and elevated expression of several growth factors are key mediators of DR. Of particular importance is VEGF which has been shown to be elevated in DR, resulting in pathogenic changes to the retinal structure, including neovascularization.[38, 39]. Given that VEGF is a predicted target of several miRNAs, including those that have already been implicated in other diabetes complications (miR-200b/c, miR-29a/b/c, and miR-93). It would be interesting to determine whether these miRNAs may also be relevant in DR[34].In the present study, all diabetic groups exhibited significantly higher body mass index when compared to control subjects, so it is of importance to consider the impact of obesity on development and progression of microvascular complications in diabetic patients. These results were in agreement with Locatelli et al., 2006 & Rossi et al., 2010[40, 41]. In the current study 55.6% of total diabetic patients had neuropathy, 30.5% of total diabetic patients had diabetic nephropathy and 25% of total diabetic cases had retinopathy.This finding is supported by many earlier studies.[42, 43]. The presence of nephropathy was significantly associated with disease duration. This result was in agreement with previous reports by Unnikrishnan et al., 2007[44]. Albuminuria may reflect underlying inflammation, expression of vascular damage, hypertension, renal endothelial dysfunction[45], and therefore, it has become clear that albuminuria is not only indicator for diabetic renal disease, but also for progress to more advanced stages of the disease[46]. In this study the presence of retinopathy was significantly associated with disease duration. This result was in agreement with previous reports by Rema et al 2002[47]. Zehetner et al., 2013 reported that retinopathy was related to duration of the disease, and it is related to poor glycemic control, normalizing blood glucose over time can significantly reduce one’s risk of developing advanced stages of retinopathy[48].The dyslipidemia of diabetes is associated with the increased cardiovascular, microvascular complication found in type "2" diabetes. In the current work is a proof that lipid abnormalities remain common in both diabetic groups. The dyslipidemia is mainly due to the insulin deficiency and metabolic abnormalitie. This result was in agreement with Chahil & Ginsberg, 2006[49].In this study there was association between miRNA 192 and disease duration; there was significant positive correlation between miRNA 192 expression levels and blood glucose, HbA1c, creatinine and urinary albumin excretion rate in diabetic nephropathy subgroup. There was a significant positive correlation between miRNA 192 expression levels and blood glucose, HbA1c in newly diagnosed DM cases and in patients with diabetic retinopathy. The study concluded that increase expression of miRNA 192 in type 2 DM, metabolic abnormalities, poor glycemic control and obesity, are risk factors related to prevalence of renal and ocular complications among diabetic subjects. Our results suggest that miRNA 192 may serve not only as molecular signatures in diabetic micro vascular complications but also as early indicators of alterations in specific biological processes in the kidney, retina, and nervous system. We recommend further studies to evaluate the diagnostic, prognostic, value of miRNA 192 and the use of anti-miRNA 192 as therapeutic in various diabetic microvascular complications.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML