-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(4): 89-92
doi:10.5923/j.ajb.20130304.01
Biological Control of Striga hermonthica Del. Bendth: Screening for Bacteria Scavenging Strigol
Hiba A. Ali1, Hassan B. Elamin1, Hamid A. Dirar2, Abdel Moneim E. Sulieman3
1Commission for Biotechnology & Genetic Engineering, National Centre for Research, Ministry of Science and Technology, Khartoum, Sudan
2Faculty of Agriculture, Department of Botany and Agric. Biotechnology, University of Khartoum, Khartoum, Sudan
3Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia
Correspondence to: Abdel Moneim E. Sulieman, Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Addition of bacterial suspensions to the root exudates of the host plant (Sorghum bicolor) significantly produced reduction in the ability of the Sorghum root exudates to induce the germination of Striga seeds. Bioassaying the treated root exudates using Striga seeds gave up to 100% reduction in germination. Most active were bacteria labelled B obtained from Safra Gadarif Sorghum strain. The germination of Striga seeds was inhibited by up to 100%. The active bacteria B could be applied as dry seed treatment before planting. Growth of the bacteria with production of the active enzyme could result in germination stimulant being destroyed as fast as it is produced. Bacteria B showed pronounced activity against the haustoria induction stimulants which was reflected on the number of haustoria formed. Bacteria B were found to belong to genus Pseudomonas.
Keywords: Biological Control, Growth Stimulants, Microorganisms, Noxious Weeds, Striga, Strigol
Cite this paper: Hiba A. Ali, Hassan B. Elamin, Hamid A. Dirar, Abdel Moneim E. Sulieman, Biological Control of Striga hermonthica Del. Bendth: Screening for Bacteria Scavenging Strigol, American Journal of Biochemistry, Vol. 3 No. 4, 2013, pp. 89-92. doi: 10.5923/j.ajb.20130304.01.
Article Outline
1. Introduction
- The genus Striga comprises parasites on many cereals and legumes crops and causes considerable yield losses of various crops in tropical and subtropical countries[1]. These hemiparasites infest two-thirds of arable land Africa and constitute the biggest single biological cause of crop damage in the continent[2]. In countries such as Ethiopia and Sudan, losses of 65 - 100% are common in heavily infested field. To germinate, Striga seeds must go through an after-ripening process, and then pre-treatment (conditioning) in a warm moist environment for several days, and finally exposure to exogenous germination stimulants. These include germination stimulants, haustoria induction stimulants and development stimulants[3][4]. The nature of these chemicals was first demonstrated by Cook et al.[5] who reported the isolation of (+)-strigol (1) from the roots of cotton (Gossypium hirsutum L.), a non-host plant which nevertheless has a strong stimulatory effect on Striga seed germination. Cotton seedlings showed average strigol production levels of 15 pg/plant/day, with peak daily production of 30 pg/plant around days 5–7[6]. In another study four sesquiterpene lactones that share structural features with the lactone rings of strigol were shown to stimulate Striga seed germination at concentrations comparable to those of strigol[7]. It was later shown[8] that strigol was the main germination stimulant secreted by a number of the most commercially important Striga hosts, including maize (Zea mays L.) and millet (Pennisetum spp.). Strigol has also been identified in the root exudates of other cereals e.g. Sorghum bicolor L. and several dicotyledonous plants including Menispermum dauricum DC[9]. Classically the strigolactones have been described as sesquiterpene lactones[10]. The role played by molecules of the strigolactone family in stimulating the germination of seeds of parasitic weeds of the genera Striga, orobanche and Alectra has never been clearly elucidated[2]). However, little was known about their biogenesis until very recently, when a study by Matusova et al., (2005) established that (+)-strigol is in fact a product of the carotenoid biosynthetic pathway.The objective of this work was to isolate naturally occurring microorganisms that could scavenge strigol as it exudes from the host plant root before it reaches the Striga seed. Our basic idea originated from a generally accepted microbiological rule that there is no naturally occurring organic substance that cannot be destroyed by some microorganism.
2. Materials and Methods
2.1. Bioassaying
- Preconditioned (at 25℃ for 10 days) sterile Striga seeds (Shambat 91) were used to monitor the effect of different exudates collected from Sorghum bicolor (variety Abu Sabeen) as plant material source of Striga germination stimulants, and to study the effect of diverse types of bacteria on those exudates. Bacteria tested for their activity against Striga germination stimulants, were obtained from soaked Sorghum seeds (3 - 10 days). Bioassaying was carried out by applying the preconditioned Striga seeds in filter paper discs and damped with the tested exudates (control or treated with bacteria) for 24 hr at 33℃.Germination of Striga seeds was counted as a percentage under microscope. Germination % was calculated as the percentage of Striga seeds induced to germinate in relation to the total number of Striga seeds per disc.
2.2. Cup Experiment
- Fifteen of sterilized Sorghum seeds were planted in each ice-cream cup, which was initially packed with sterilized sand. These were finally thinned to 3 - 4 seedlings per cup. Buchnnar funnel was used to extract exudates, under water pump pressure, from host roots after 7 days of growth[11]. In control experiment ice-cream cups were irrigated with sterile distilled water for 7 days before extraction. Treated ice cream cups were irrigated with suspension of different bacteria in sterile distilled water for 7 days before extraction. Exudates extracted from both controlled and treated cups were bioassayed to test the effect of each bacteria studied on the host root exudates.
2.3. Pot Experiment
- Nine pots of 9cm in diameter were prepared and packed with mixed soil made up of sand + clay. These were then sterilized by heating in an oven at 160℃ for 3 hr. The sterile soil of the prepared pots were seeded with 0.03 gm of Striga seeds. Abu Sabeen was then planted at different ratios not exceeding 20 seeds per pot, these were later thinned after 7 days to only 2 seedlings per pot[11].Pots were then divided into 3 groups according to their irrigation methods: Group I, was irrigated with sterilized tap water for 45 days, Group II, was irrigated with sterilized tap water + bacteria suspention (B) from Safra Gadarif soaked seeds, for 45 days, and Group III, was irrigated with sterilized tap water + bacteria suspention (E) from Abu Sabeen soaked seeds, for 45 days.
2.4. Thin Layer Chromatography (TLC)
- Exudates obtained from different treatments and control experiments were pooled in parts and concentrated using rotor evaporator under reduced temperature and pressure. 20 ml of each exudate was subjected to physical fractionation using separating funnel against equal volume of ethyl acetate [8]. The ethyl acetate fractions were pooled and concentrated to be examined using TLC.Standard chromatograms of the extracted stimulants were prepared by applying 20-µl solution (5 mg/ml) to a silica gel plate and developing it in different solvent systems depending on the type of extract. These include toluene: ethyl acetate: formic acid (5:4:1), DCM: MeOH (9:1) and different ratios of H2O: MeOH for RP silica gel. Chromatograms were detected under UV light (254 and 366) and sprayed with diagnostic reagents, which include: vanillin-H2SO4 reagent and Natural Product Reagent.
3. Results and Discussion
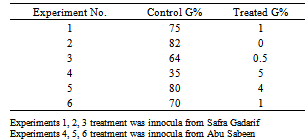
3.1. Effect of Bacteria Inocula on Striga Germination
- The addition of bacteria suspensions to the root exudates of the host plant (Sorghum, Abu Sabeen) significantly produced reduction in the ability of the exudates to induce the germination of Striga seeds. Results of bacteria suspensions studied on the germination (G%) of Striga are summarized in Table (1). Bioassaying the treated root exudates using Striga seeds gave up to 0% germination.These results confirm the idea of existence of microorganisms that are able to abolish the activity of strigol and its analogues. Exudates from Sorghum roots were previously reported to contain analogues of strigol e.g. sorgolactone and they are known collectively as strigolactones[12][13]). The lactone rings, the linkage between them and the substituents on them, seem to be of crucial importance for the germination stimulant activity [7][13]). The terminal lactone ring of the strigol and its analogues are reactive and instable. They could readily be inactivated by non-specific esterase enzymes, apparently by hydrolysing them resulting in their loss of stimulant activity [13]. It is possible that the studied bacteria produce esterases or more specific lactonases that inactivated strigol lactones.
|
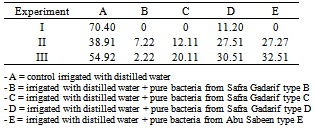
3.2. Effect of Pure Bacteria Isolates on Striga Germination
- Different bacteria species were isolated from the innocula obtained from soaked seeds of Safra Gadarif and Abu Sabeen. Results of the effects of suspensions of these isolates on the germination of Striga seeds are summarized in Table (2).Most active were bacteria labelled B and C obtained from Safra Gadarif strain. The germination of Striga seeds was inhibited up to 100%. Inoculum C was not studied further because it was not obtained in 100 % pure form.In another experiment the active bacteria B was used as dry seed treatment and the results of this experiment were summarized in Table (3). Bacteria B showed strong activity against Sorghum root exudates when bioassayed using Striga seeds. This show that active bacteria could be applied as dry seed treatment before planting in the same way as Rhizobium is applied to legume seeds. Growth of the bacteria with production of the degradative enzyme could result in germination stimulant being destroyed as fast as it is produced. Germination of Striga seeds might be selectively prevented or slowed down.
|
|
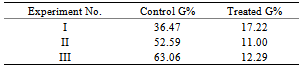
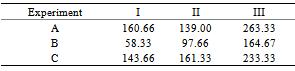
3.3. Effect of Bacteria on Haustoria Formation
- Bacteria innocula and pure isolate (bacteria B) were tested for their activity against haustoria induction stimulant, and the results are summarized in Table (4). Haustoria induction stimulant is the second important Sorghum root exudate which affects Striga germination cycle[12]. Bacteria B showed pronounced activity against the haustoria induction stimulants which was reflected on the number of haustoria formed.
|
3.4. Characterisation of Bacteria B
- Isolates of bacteria B, which were proven to be active against different Striga germination stimulants from Sorghum root exudates, were subjected to different microbiological tests with an attempt of identifying it. Bacteria studied were found to be blue-greenish in colour which was clearly reflected in the growing media. Other characteristics of the studied bacteria included: rod shaped; Gram negative; non sporing; motile; Catalase positive and Oxidase positive. The studied bacteria were suspected to be a member of genus Pseudomonas.
3.5. Chromatography of the Root Exudates of Sorghum
- The TLC of the ethyl acetate fraction from control exudates showed a range of phenolic compounds when studied using polyamide and C18 silica plates under short wave UV light. Pronounced were the major phenolic compounds when sprayed with FeCl3. The TLC pattern of the ethyl acetate fraction of the treated root exudates was different from those of the control as far as the major phenolic compounds were concerned. This shows that the major phenolic compounds of the treated root exudates fraction were subjected to chemical changes involving different sites within their chemical structures. This again confirms the effect of the bacteria on the stimulant produced by Sorghum roots.
4. Conclusions
- Addition of bacteria suspensions to the root exudates of the host plant (Sorghum, Abu Sabeen) significantly reduced the ability of the exudates to induce germination of Striga seeds. Bioassaying the treated root exudates using Striga seeds gave up to 0% germination.Most active were bacteria labelled B and C obtained from Safra Gadarif Sorghum strain. The germination of Striga seeds was inhibited up to 0%. Innocula of bacteria C was not studied further because it was not obtained in 100 % pure form. The active bacteria B could be applied as dry seed treatment before planting as Rhizobium is applied to legume seeds (Table 1). Growth of the bacteria with production of the degradative enzyme could result in germination stimulant being destroyed as fast as it is produced. Bacteria B showed pronounced activity against the haustoria induction stimulants which was reflected on the number of haustoria formed.Bacteria B were found to belong to the genus Pseudomonas, and attempts are made to classify them down to the species level. Further bacterial isolates together with different plants extracts are under similar tests with the aim of biologically control of Striga weeds.
ACKNOWLEDEGEMENTS
- Our sincere thanks go to all collaborators in the field, especially the late Prof. Larry Butler and Prof. Gebisa Ejeta, Purdue University, Indiana State, USA, who gave us invaluable information concerning this project. Thanks also go to Mr. Alsamani, Department of Botany, Faculty of Agriculture, University of Khartoum, for his assistance in all experiments involved in this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML