-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(3): 84-88
doi:10.5923/j.ajb.20130303.04
Acute Toxicity of Solanum macrocarpon Linn (Solanaceae) on Wistar Rats: Study about Leaves and Fruits
Victorien Dougnon1, 2, Honoré Bankolé2, Patrick Edorh1, 3, Jean Robert Klotoé2, Jacques Dougnon2, Lauris Fah2, Frédéric Loko2, Michel Boko1
1Laboratory of Toxicology and Environmental Health, Interfaculty Center of Formation and Research in Environment for the Sustainable Development, University of Abomey-Calavi (UAC), 01 BP 1463 Cotonou, Benin
2Laboratory of Research in Applied Biology, Polytechnic School of Abomey-Calavi, University of Abomey-Calavi, 01 BP 2009 Cotonou, Benin
3Department of Biochemistry and Cellular Biology, Faculty of Science and Technology, University of Abomey-Calavi (UAC), 01 BP 526 Cotonou, Benin
Correspondence to: Victorien Dougnon, Laboratory of Toxicology and Environmental Health, Interfaculty Center of Formation and Research in Environment for the Sustainable Development, University of Abomey-Calavi (UAC), 01 BP 1463 Cotonou, Benin.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
S. macrocarpon is a highly consumed vegetable in Benin with values recognized by herbal medicine.The objective of this study was to assess its acute toxicity on Wistar rats. Fruits and leaves were shade dried, powdered, boiled and filtered. The powders obtained from leaves and fruits were orally administered to randomly selected animals divided into five groups treated with saline, 300 mg/kg and 2000 mg/kg of powders. The anomals were observed along 14 days focusing attention on different behavior manifestations. Body weight, hematological (Complete Blood Count) and biochemical analyses (urea, creatinine and transaminases) were conducted. About S. macrocarpon’s leaves, the dose of 300 mg/kg resulted in the death of no rat. No mortality was recorded at the dose of 2000 mg/kg. It was the same for the fruit powder. Powders of leaves and fruits of S. macrocarpon then have an LD50 greater than or equal to 5000 mg/kg. There was a significant increase in the weight of rats after administration of powders of S. macrocarpon. Powder of S. macrocarpon has not resulted in a weight loss of Wistar rats but weight gain is not due to the administration of a single dose of powders. No statistical difference was observed when comparing averages of different groups (p> 0.05) for both transaminases ALT and AST. Powders of S. macrocarpon induced a significant decrease in blood urea in groups II, III and IV. From the two parts used (leaves and fruits), only fruit had this effect regardless of dose. The hematological parameters were not significantly different between the groups. Biochemical and hematological assays showed that neither leaves nor fruit of this vegetable have toxicity on the rats. These results proved that fruits and leaves of S. macrocarpon can be safely consumed.
Keywords: S. macrocarpon, Medicinal Plants, Food Security, Toxicity
Cite this paper: Victorien Dougnon, Honoré Bankolé, Patrick Edorh, Jean Robert Klotoé, Jacques Dougnon, Lauris Fah, Frédéric Loko, Michel Boko, Acute Toxicity of Solanum macrocarpon Linn (Solanaceae) on Wistar Rats: Study about Leaves and Fruits, American Journal of Biochemistry, Vol. 3 No. 3, 2013, pp. 84-88. doi: 10.5923/j.ajb.20130303.04.
Article Outline
1. Introduction
- S. macrocarpon is a plant of the same family as tomatoes, peppers: eggplant family called Solanaceae[1]. The botanical genus includes herbaceous plants or shrubs leaves usually alternate, sometimes opposite. The inflorescence is a cyme, usually single seed. The flowers are hermaphrodite, with star-shaped corolla with five petals often reflected (back to back). The protruding stamens form a cone around the gynoecium. The ovary is superior. The fruits are berries[1]. Also called S. macrocarpum, it is used as a vegetable in many countries in West Africa[2-3]. S. macrocarpon is also used for its therapeutic properties. Despite this dual purpose, little scientific data on the knowledge of the plant species exist.Although studies have been conducted on the aqueous extract of S. macrocarpon[2], no data on the toxicity of powders of leaves and fruits are available in Benin. Since there is in the world almost 1500 species belonging to the genus Solanum[4], preliminary studies have shown that the Benin variety of S. macrocarpon has a similar nutritional value to Moringa oleifera, a vegetable heavily used in the nutritional recovery of children and people with compromised immunity[5-6] in Benin. Unfortunately, the presence of certain chemical compounds such as alkaloids, especially in fruits, fears its promotion as a dietary supplement. That is why an earlier study was to evaluate the cytotoxicity at short and medium term of powders of leaves and fruits of S. macrocarpon[7]. The results suggested that the leaves and fruit vegetable showed no risk and that its consumption could be promoted. However, it is important to make more extensive toxicity studies on Wistar rats (model physiologically similar to humans) to investigate the effect of S. macrocarpon on hematological parameters, liver and kidney to further reassure the scientific world. This study is then to complete cytotoxicity tests performed on larvae shrimp (Artemia salina) and will confirm or not the harmlessness of the empirical use of S. macrocarpon as food and medicine.
2. Material and Methods
2.1. Material
- The mature leaves and fruits of S. macrocarpon were the plant material used in this study. It were purchased in July 2012 on the site of Houeyiho located at 6° 21' 20'' North latitude and 2° 21' 35'' East longitude in Benin. It is the biggest site of Benin and the soil is enough fertile[8]. Wistar albino rats were bred in the Laboratoire de Recherche en Biologie Appliquée (LARBA), University of Abomey-Calavi (Benin) at constant temperature of 22 ± 1°C with a cycle of 12 hours at light and 12 hours of darkness. They were fed with granulated provender and water ad libitum.
2.2. Methods
- The leaves were thoroughly washed with distilled water (with a few drops of bleach) and then dried at laboratory temperature of 16°C in LARBA for 17 days while fruits were also washed and finely cut into small pieces before being dried for 09 days. Leaves and dried fruits were ground for ten minutes using a Moulinex Sayona. The powders were then sieved using sieves of 0.2 mm and stored in sterile containers until needed.
2.2.1. Toxicity Tests
- The tests were performed according to OECD Guideline for testing of chemicals through the method 423 (OECD, 2001). The principle of this test is that, with only a sequential process, using a minimum number of animals per step, information on the acute toxicity of the substance are obtained and is sufficient for the purposes of classification. A fixed dose of the substance is orally administered to a group of animals.The absence or presence of mortality related to the substance in a group will determine the next step, ie:− Stopping test− Administration of the same dose to three additional animals,− Administration of the dose immediately above or below to three additional animals.At the beginning of the test, the rats aged of 12 weeks and weighing between 140 and 170 grams were randomly selected, marked to permit individual identification, and kept in their cages for acclimatization to the laboratory conditions for five days before the experiment. Powders of leaves and fruits were dissolved in physiological saline and administered to rats at doses of 1 ml / 100 g of body weight. It were given in a single dose using a feeding tube. The rats were fasted overnight but with access to water. Each step required three animals. The initial level of 300 mg/kg was chosen. Since it is a vegetable which was used, from four doses proposed by the Directive 423, it is the one for which it might expect to observe mortality among some of the treated animals. Thus, the rats were divided into different lots. The control lot received no treatment while lots I and II were respectively given 300 mg/kg and 2000 mg/kg of powdered leaves. Lots III and IV were respectively given 300 mg/kg and 2000 mg/kg of fruit powders. The animals were observed individually at least once during the first 30 minutes, periodically during the first 24 hours after treatment. Particular attention was observed for 14 days after administration of the substance. All observations were recorded systematically, individual records being maintained for each animal. Attention has focused in particular on the observation of the various manifestations of tremors, convulsions, salivation, diarrhea, lethargy, sleep and coma. The following parameters were investigated:− Body WeightIndividual weight of each rat was determined shortly before administration of the powders. At the end of the test, the animals were weighed and then sacrificed.− PathologyBlood samples were performed on all rats for Complete Blood Count and biochemical (urea, creatinine, transaminases) parameters in LARBA. The retro-orbital sampling was adopted. The Complete Blood Count was to determine the number of red blood cells (NGR), the number of white blood cells (WBC), hemoglobin (Hb), hematocrit (Hte), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin Concentration (MCHC), Mean Corpuscular Concentration in hemoglobin (MCHC) and platelet count. These tests were performed using a Sysmex KX-N21 according to the methodology of Sodipo et al.[2]. Urea, creatinine and transaminases were assayed by the kinetic method in accordance with the methodology of Sodipo et al.[2].
2.2.2. Statistical Analysis
- Means and standard deviations were compared with a significance level of 5%. Using the nonparametric Mann-Whitney test, the average of the control lot were compared to those of Lots I, II, III and IV and those of lots I, II on the one hand and III, IV on the other hand were compared. XL Stat 2011 software was used for statistical tests while Microsoft Excel 2013 has permitted the creation of graphs.
3. Results and Discussion
3.1. Determination of LD50
- About S. macrocarpon’s leaves, the dose of 300 mg/kg resulted in the death of no rat. No mortality was recorded at the dose of 2000 mg/kg. It was the same for the fruit powder. Powders of leaves and fruits of S. macrocarpon then have an LD50 greater than or equal to 5000 mg/kg[9]. Nevertheless, a certain lethargy was noted in treated rats. Plants with LD50 above 1000 mg/kg by oral route are safe or of low toxicity[10]. These results confirm previous work done on the same substances[7]. Lethargy observed in rats could be due to stress caused by handling.
3.2. Effect Powders of S. macrocarpon on Weight Gain in Wistar Rats
- There was a significant increase in the weight of rats after administration of powders of S. macrocarpon. Powder of S. macrocarpon has not resulted in a weight loss of Wistar rats (Figure 1). However, the variation is similar between lots; so weight gain is not due to the administration of a single dose of powders. As the purpose of this study was just to evaluate the in vivo toxicity of powders, it would be interesting to explore the role of these powders on weight gain in malnourished children or people exactly as other authors did it on Moringa oleifera[5].
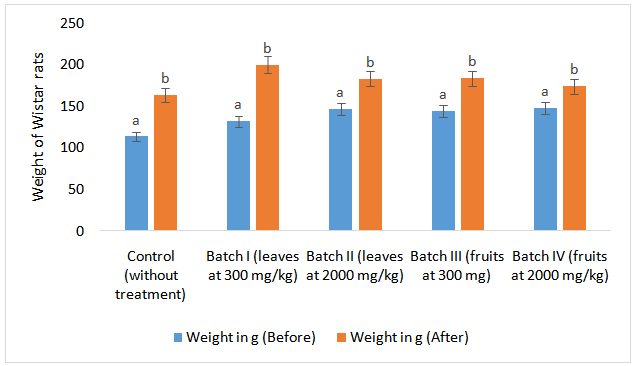 | Figure 1. Weight’s variation on Wistar rats |
3.3. Exploration of Liver Damage
- No statistical difference was observed when comparing averages of different groups (p> 0.05) for both transaminases ALT and AST (Figure 2).
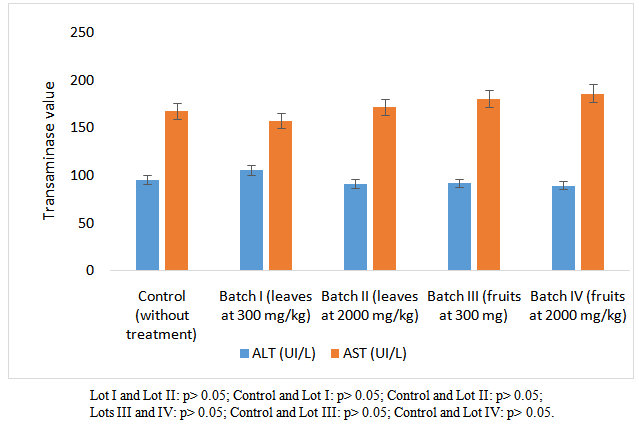 | Figure 2. Variation of transaminases on Wistar rats |
3.4. Exploration of Renal Parameters
- Powders of S. macrocarpon induced a significant decrease in blood urea in Lots II, III and IV. From the two parts used (leaves and fruits), only fruit had this effect regardless of dose (Figure 3). The fruits of S. macrocarpon therefore have a better effect on renal function[4],[2] than leaves that regulates urea until a dose of 2000 mg/kg. This situation was further tested in the assay of creatinine. Only leaves powder at 2000 mg/kg induced decrease in creatinine, especially when compared to the lot receiving leaves powder at 300 mg/kg.
3.5. Exploration of Hematological Parameters
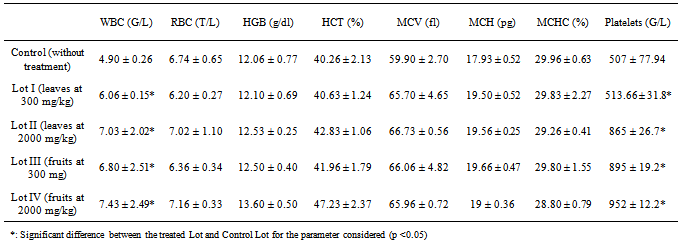
- White blood cells and platelets were significantly elevated (Table 1). From these two parameters, a possible witness trombocytose coupled with leukocytosis can be explained by the use of nasogastric tube metal. Indeed, rats of test group were overfed while the control group had free access to water. The material used to feed rats of test groups may have abused the esophageal wall of these animals, causing acute inflammatory syndrome. As the other parameters have been no significant difference, this explanation is reinforced.
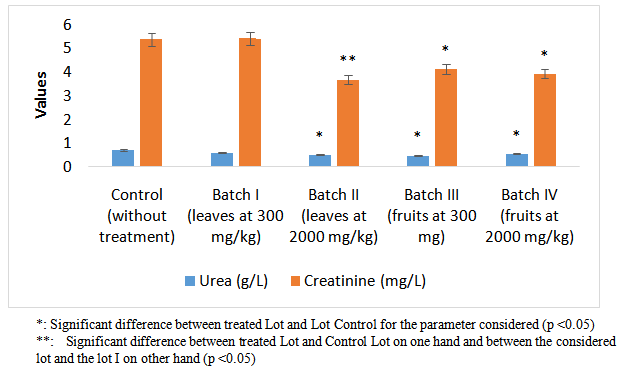 | Figure 3. Variation of urea and creatinine on Wistar rats |
|
4. Conclusions
- The present study revealed that leaves and fruits of S. macrocarpon has no toxicity on biological parameters. S. macrocarpon is safe for consumption, showing no liver or renal acute toxicity. Thus its medical properties should be explored and assessed.
ACKNOWLEDGMENTS
- The authors gratefully acknowledged the valuable contributions of The National Program of Traditional Medicine and Pharmacopoeia of Benin which support this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML