-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(3): 80-83
doi:10.5923/j.ajb.20130303.03
Physicochemical, Chemical and Microbiological Characteristics of Vinasse, A By-product from Ethanol Industry
Ahmed O.1, Abdel Moneim E. Sulieman2, Sirelkhatim B. Elhardallou3
1Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira, Wad-Medani, Sudan
2Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia
3Faculty of Applied Medical Sciences, University of Taif, Taif, Kingdom of Saudi Arabia
Correspondence to: Abdel Moneim E. Sulieman, Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ethanol production through sugar juice or final molasses fermentation yieldsvinasse as a by-product. The objective of this study was to evaluate the quality of vinasse byproduct of Kenana Ethanol Factory through determination of some of its, physicochemical, chemical and microbiological characteristics. At temperature 28.3˚C, vinasse gave brix value of 11.01˚Bx, colour (74061 M.A.U), and conductivity (22.90μ s/cm). The proximate chemical analysis showed high contents of moisture (82.27 %), ash (10.60%), protein (6.20%), and very low carbohydrate content (0.93%). The total dissolved solids and total suspended solids found in vinasse were, 10500 mg/l, and 4633.3 mg/l, respectively. Vinasse had a very high biological oxygen demand and chemical oxygen demand values, which were 36666.67 and 117000 mg/l, respectively. Vinasse had different amounts of minerals, calcium (1734.67 mg/l) (macro mineral), however, its contents of copper, iron, manganese, and aluminium (micro mineral) were found to be 86, 17, 14, and 0.01mg/l, respectively. In addition, vinasse samples were devoid of all microbial groups. Based on the results it is recommended to use vinasse effluents as a raw material to produce fertilizers, and drying to powder to add as an animal feed ingredient.
Keywords: KeywordVinasse, Sugarcane, Ethanol, Physicochemical Characteristic
Cite this paper: Ahmed O., Abdel Moneim E. Sulieman, Sirelkhatim B. Elhardallou, Physicochemical, Chemical and Microbiological Characteristics of Vinasse, A By-product from Ethanol Industry, American Journal of Biochemistry, Vol. 3 No. 3, 2013, pp. 80-83. doi: 10.5923/j.ajb.20130303.03.
Article Outline
1. Introduction
- Ethanol refers to a type of alcohol consisting of two carbon atoms, five hydrogen atoms, and one hydroxyl group. Asopposed to gasoline, ethanol is a pure substance consisting of only one type of molecule: C2H5OH. In ethanol production, however, it is necessary to distinguish anhydrous ethanol(or anhydrous ethyl alcohol) and hydrous ethanol (or hydrous ethyl alcohol). The difference lies in the water content of the ethanol grade: while the water content of anhydrous ethanol is approximately 0.5 percent by volume the hydrous ethanol that is sold at fuel stations has water close to 5 percent by volume. In the industrial production of ethanol, the hydrous grade is the one that comes directly from the distillation tower. Producing anhydrous ethanol requires an additional processing stage that removes most of the water contained in the fuel[1]. The world is now producing a huge amount of ethanol (ethyl alcohol) through the fermentation of agricultural materials or molasses from sugar industry followed by separation of the formed ethanol by distillation process. The distillation process produces highly polluting residue called vinasse, stillage, or slops. Different ways of utilization treatment and final disposal of vinasse have been developed to avoid its negative impacts on the environments.Vinasse, a residual substance left after sugarcane alcohol distillation, represents a major environmental problem for the ethanol industry. No one has found a convenient and economical disposal solution for this black-reddish, viscous, and high BOD vinasse. The disposal of vinasse represents a major environmental problem due to its high BOD. This material can cause damage to aquatic life, especially when dumped in large volume in rivers, streams, and landfills[2].The word vinasse is derived from the latin word vinacaeus, originally known as yeast wine, its use, reported in several tropical countries and in Europe, is as an additive or feeding supplement for the feeding of ruminants and non-ruminants. Additionally it has been used as a fertiliser since the beginning of the 20th century.Vinasse is classified as a class II residue, not inert but not dangerous. The chemical composition of sugarcane ethanol vinasse is variable and depends of the wine raw materials. The wine characteristics depend also of the must preparation, alcoholic fermentation system, type of yeast, distillation and separation[3].The objective of the present study was to evaluate the quality of vinasse by-product of Kenana Ethanol Factory (Central Sudan) through determination of its chemical composition, physicochemical properties, and microbiological characteristics.
2. Materials and Methods
2.1. Materials
- Vinasse samples were collected from Kenana Sugar Company during the production season 2010. Ethanol factory has been constructed near the sugar factory to make use of the molasses by-product in production of ethanol. The vinasse samples were collected immediately after drainage from the ethanol factory pipelines.
2.2. Physical and Physicochemical Properties of Vinasse
- The physical properties of vinasse were determined according to ICUMSA[4].The total soluble solids colour of vinasse were determined by using hand Refractometer and Talameter device, respectively. While both the conductivity and the total dissolved solids (TDS) of vinasse were measured using a Conductivimeter. On the other hand, the total suspension solids (TSS) was measured using Spectrophotometer “Hatch. And the pH of the vinasse was measured using pH-meter (pHS-3C Digital) at ambient temperature.
2.3. Biological Oxygen Demand
- The Biological Oxygen Demand (BOD) refers to the measurement of the oxygen required when the organic waste is decomposed by bacteria. In order to determine the BOD of a sample of organic waste, water saturated with dissolved oxygen is added to the sample under test and kept at a constant temperature of 20℃ for a period of five days in a closed incubation bottle[5].
2.4. Chemical Oxygen Demand
- The chemical Oxygen Demand signifies the amount of oxygen required, when the sample under test is completely decomposed by a strong chemical oxidizing agent like, permanganate[6].Chemical Oxygen Demand of vinasse was measured using a Photometer Device according to AOAC[7] method.
2.5. Proximate Chemical Composition of Vinasse
- The vinasse contents of moisture, ash, protein and carbohydrate were determined according to AOAC[8] method.
2.6. Determination of Minerals and Mineral Salts
- The vinasse contents of calcium, copper, iron, manganese, aluminium, sulphate, phosphate, and nitrate were determined using a photometer according to AOAC[9].
2.7. Total viable Count
- The total viable count of vinasse was enumerated by culturing one ml of vinasse on nutrient agar medium following the method of APHA[10]. Incubation was accomplished at 30℃ for 48 hours.
2.8. Yeast and Mould Count
- The yeast and mould strains were enumerated by culturing one ml of vinasse on potato dextrose agar (PDA) medium and incubating for 72 hours at 25℃ (APHA,[10].
2.9. Thermophilic bacterial Count
- Thermophillic bacteria was enumerated by culturing one ml of vinasse on Dextrose Trypton Agar and confirmed by culturing on Reinforced Clostridium Agar, and also on Iron Sulphate Agar. Incubation was accomplished at 50-55˚C for 72 hours[11].
3. Results and Discussion
3.1. Physical and Physicochemical Properties of Vinasse
- Table (1) shows some of the physical properties of vinasse. The temperature, brix, colour, and conductivity values were 28.33℃, 11.02 Bx, 74061 M.A.U, and 22.90μ s/cm, respectively. Ethanol vinasse is a dark, brown colour liquid, of acid nature, that remains after alcoholic distillation at 107ºC as indicated by Wilkieet.al,[12], however, Marional et.al,[2] reported that vinasse is a light brown liquid.
|
|
3.2. Proximate Chemical Composition of Vinasse
- Figure (1) shows the proximate chemical composition of vinasse. The results show that vinasse contained high moisture content (82.27%), ash (10.60%), proteins (6.20%), and very low carbohydrates content (0.93%). According to Marional et.al,[2] vinasse is consisting basically of water (93%). Yeast residues will lead to the presence of amino acids and proteins in the wastewater[14]. In Brazil, vinasse produced from juice and molasses, has an ash content of 15.292% and 19.879%, respectively as found by Polack et.al.,[15]. However, Harper[13]reported that ash content of vinasse produced from molasses, cassava, and sorghum was 10.70, 10.50, and 6.10 mg/l, respectively, while the carbohydrates content were 8.00, 20.10, and 3.40 mg/l, respectively.On the other hand, the chemical composition of sugarcane ethanol vinasse is variable and depends on wine raw materials. The wine characteristics depend mostly on the preparation, alcoholic fermentation system, types of yeast, distillation and separation as reported by Arnoux and Michelot[16].
3.3. Minerals and Mineral Salts
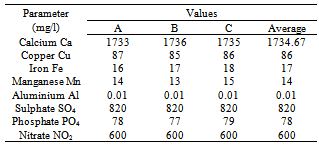
- Vinasse had different amounts of minerals, calcium (1734.67 mg/l) (macro mineral), on the other hand its contents of copper, iron, manganese, and aluminium (micro mineral) were found to be 86, 17, 14, and 0.01mg/l, respectively, as shown in Table (2). However, vinasse produced in Brazil from juice and molasses, had low calcium content (408, and 714 mg/l) respectively, Polack et.al, (1981) reported that in Australia vinasse produced from molasses contain low calcium content (1.121 mg/l).Table (2) also shows that the amount of sulphate, phosphate, and nitro compounds present in vinasse, were found to be 820, 78, 600 mg/l, respectively. Presence of these mineral salts could be attributed to unused salts by the yeast during fermentation to produce ethanol, which was added as nutrient of yeast to increase its activity.
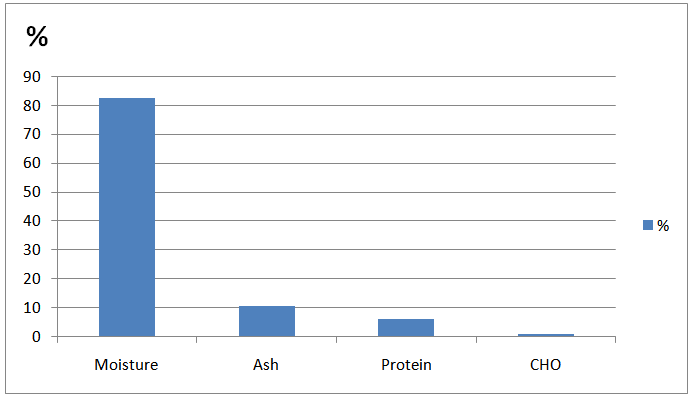 | Figure 1. Proximate Chemical Analysis of Vinasse |
3.4. Microbial Characteristic of Vinasse
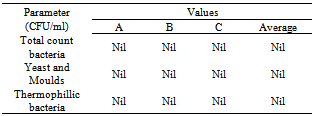
- As shown in Table (2), different microbial groups were not detected in vinasse samples. This could be attributed to the production of vinasse at high temperature (105-110˚C) which is intolerable for microbial growth. Also thermophillic bacteria can`t grow because vinasse containing a considerable amount of alcohol, which is known to be strong inhibitor for most of the microorganisms.
4. Conclusions
- Based on the results it could be concluded that most of the physicochemical components of vinasse vary considerably. Vinasse had high value of biological oxygen demand and chemical oxygen demand, this indicates its high organic matter content, the high organic matter content causes heavy pollution of water and soil if the residues are being disposed untreated. Appreciable amounts of minerals and mineral salts were found in vinasse, indicating that it can be used as raw material to produce fertilizers like urea. In addition, vinasse can be used as a raw material to produce animal feed, as it is free from microorganisms. However, to solve the problem of environmental pollution as a result of the high contents of BOD and COD, it is recommended to use vinasse effluents as a raw material to produce fertilizers, concentration to syrup or spray drying to a powder and to use as animal feed ingredients.
ACKNOWLEDGEMENTS
- The authors express their gratitude to the administration, staff members and technicians of the Kenana Ethanol Factory, Sudan for the encouragement and assistance in conducting this research.
References
| [1] | Anon. (2002). Third estimate of world molasses production 2001/02. F.O. Licht’s World Ethanol & Biofuels Report. July 15. |
| [2] | A.P. Marianol, S.H.R. Crivelaro, D. D. De Angelis andD.MBonotto (2006). “Use of Vinasse, an Ethanol Distillery Waste, as Anamendment to Bioremedation of Diesel oilcontaminated Soils” Interamerican Confederation of Chemical Engineering (IACCHE). |
| [3] | M. Arnoux and E. Michelot(1988).Méthanisation des vinasses de mélasse de canne à sucre par le procédé SGN à film fixe à la Societéindustrielle de sucrerie – Guadeloupe. EU Direction GénéraleÉnergie, Brussels (Belgium). |
| [4] | ICUMSA (International Commission Uniform Methods of Sugar Analysis) (1998), (ICUMSA) Method Book with First Supplement. |
| [5] | S. Lenore, E.Clescerl, E. Greenberg and D. Andrew (1999). Standard Methods for Examination of Water & Wastewater (20th ed.). Washington, DC: American Public Health Association. ISBN 0-87553-235-7. Also available by online subscription at www.standardmethods.org |
| [6] | N. Clair, L. Sawyer and F. Parkin (2003). Chemistry for Environmental Engineering and Science (5th ed.). New York: McGraw-Hill. ISBN 0-07-248066-1. |
| [7] | AOAC, (1984) Association of Official Chemists, Agricultural Official methods of analysis, 14th ed. Washington, D.C. |
| [8] | AOAC (2000). Official Methods of Analysis (17th edition) Associationof Official Analytical chemists, Arlington, VA, USA. |
| [9] | AOAC. (1980). Official Methods of analysis. Helrich k(ed.) 15thedn. Association of Analytical Chemists, Inc. USA pp. 777. |
| [10] | APHA (1967).Lin: speck. Ml. (Ed). Compendium of Methods for the Microbiological Examination of Foods. American. Public Health Association, Washington, D.C. |
| [11] | M.Cheesbrough, (1984), Medical Laboratory Manual for Tropical countries. Volume II. |
| [12] | A.C. Wilkie, J. Kelly and M.Owens (2000) “Stillage characterization and Anaerobic Treatment of Ethanol Stillage From Conventional and Cellulosic Feed-Stock” Soil and Water Science Department, PO Box 110960, University of Florida, Gainesville, FL 32611-0960, USA, Biomass and Bio energy 19 page 63-102. |
| [13] | G.L. Harper(1980). Combustion System Development for Firing of Pulverized Bagasse, M.Sc. Thesis, Mechanical Eng. Dept., Louisiana State University, Baton Rouge, La, USA.. |
| [14] | TBW GmbH Frankfurt (1998): EndberichtzurFörderung der AnaerobitechnologiezurBehandlung kommunaler und industrieller Abwässer und Abfälle. GTZ TBWBMZ, Frankfurt (Germany). |
| [15] | J.A. Polack,D.F.Day and Y.K. Cho (1981) Gasohol from Sugarcane Stillage Disposition, Audubon Sugar Institute, Louisiana State University, September, p. 47,. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML