-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(3): 74-79
doi:10.5923/j.ajb.20130303.02
Fertilizer Application Effect on Heavy Metal Composition and Biochemical Responses of Earthworm Eisenia Fetida (Savigny) in a Sandy Farm Soil
Ukpabi C. F1, Akubugwo E. I2, Ibiam U. A3, Agbafor K. N3, Ezeigbo R. O4, Wogu C5
1Department of Biochemistry Abia State Polytechnic, Aba
2Department of Biochemistry Abia State University, Uturu
3Department of Biochemistry Ebonyi State University, Abakiliki
4Department of Biology Abia State Polytechnic, Aba
5Department of Chemistry Abia State Polytechnic, Aba
Correspondence to: Ukpabi C. F, Department of Biochemistry Abia State Polytechnic, Aba.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
We investigated the effect of inorganic and organic fertilizer application in soil at different concentrations on the pollution index per metal and exposure time on the biochemical responses of the earthworm Eisenia fetisa (Savigny).This is to elucidate the mechanism of action of heavy metals and explore the potential for using these responses as biomarkers for monitoring contaminated soils. The elemental composition of the soil and fertilizer samples was analyzed using atomic absorption spectrophotometer. Molybdemum (Mo) levels (mg/kg) rated highest in the soil (1580.00± 1.20), inorganic fertilizer (2570.00±1.73) and animal manure (1080.00± 2.00) samples while vanadium (V), arsenic (As), mercury ( Hg), silver (Ag) and chromium (Cr; only in soil), were not detected by our analytical method. The pollution assessment of the metals followed the sequence: Cd (33.34) > Cr (9.84) > Mg (9.50) > Pb (4.31) > etc and Cr (28.88) >Mg (14.46)> Cd (9.74) > Cu (4.43) > etc in the inorganic and animal treated soils respectively. The biochemical responses of the earthworms (Eisenia fetida) exposed to 0.1 and 0.5 g/kg soil application rate for 28days indicated significant (P<0.05) level (IU/I) in all the parameters measured except albumin (ALB) which showed no significant reduction (p>0.05). AST, ALP and GST showed more sensibility with significant difference (p<0.05) in concentration than ALT, ACP and CAT respectively. These biochemical responses measured in E. fetida (Savigy) could be used for chemical testing in the laboratory or for soil contamination surveys.
Keywords: Earthworms, Eisenia Fetida,Biomarkers, Heavy Metals, Inorganic and Organic Fertilizers
Cite this paper: Ukpabi C. F, Akubugwo E. I, Ibiam U. A, Agbafor K. N, Ezeigbo R. O, Wogu C, Fertilizer Application Effect on Heavy Metal Composition and Biochemical Responses of Earthworm Eisenia Fetida (Savigny) in a Sandy Farm Soil, American Journal of Biochemistry, Vol. 3 No. 3, 2013, pp. 74-79. doi: 10.5923/j.ajb.20130303.02.
Article Outline
1. Introduction
- A major agricultural research priority is to sustain soil productivity and to develop better methods to monitor changes in soil physico-chemical properties as affected by management. Evaluation of the effects of contaminants in soils has become a priority, following the abuse, use and reuse of soil for food production. Molecular markers could be used as diagnostic and prognostic early-warning tests to detect and assess the effects of pollution, particularly low concentrations of complex mixture of contaminants[1].Soil assessment methods which only determine total metal concentrations are often not accurate in predicting soil organism toxicity[2]. The toxicity test available for soil fauna have been restricted, until now to growth and reproduction. Thus sublethel invertebrate tests are needed for soil pollution testing in the laboratoryBurrowing animals, soil bacteria, fungi and the roots of plants are the part of the biological processes of soil formation[3]. Earthworms are common in a wide range of soils and may represent 60-80% of the total soil biomass[1]. This makes them one of the most suitable bioindicator of soil organisms for testing pollutants in the soil. Soil pollutants both in acute and chronic exposure intereact with many different cellular components, thereby interfering with the normal metabolic functions causing cellular injuries and on extreme cases, death of soil organisms[4]. Toxicology and biochemical investigation have led to the identification of large number of potential real target components whose normal activities are inhibited, enhance or modified by heavy metal pollutants[5]. The poisoning of heavy metals may be summarized as the effects on enzymes and on cellular macromolecules via free radical formation.The term heavy metal has been called a “Misinterpretation” in an IUPAC technical report due to the contradictory definitions and its lack of coherent scientific basis (Wikipedia, 2013). Heavy metals apply to the group of metals which are toxic or poisonous even at low concentrations and have high atomic number, number of atomic weight and a specific gravity greater than 5.0[21.The possible effect of this definition may arrange toxic metals (Cd and Pb), trace elements (Fe and Zn) and/or exclude light metals (Mg and Bo) as classes of heavy metals.In Nigeria, mineral NPK fertilizers and animal manures are the main fertilizers used by farmers. For many years fertilizers have been regulated to ensure that the product label provides accurate information on essential plant nutrient content. In recent years, concerns about quality of some fertilizer products have extended beyond nutrient contents to the potential presence of non-nutrient toxic substances such as heavy metals. The current fertilizer application recommends 3-6 bags of NPK fertilizer application of nutrients (kg/ha), follows by 1-2 bags of urea[6]. The maximum application rate of this management system is about 0.225g/kg soil/yr for low fertility soil (where, 1 hectare = 2 x 106 kg/soil).Taking the maximum application rate as 50 kg/ha/yr with multiplication factor of 1.0 million. Informal survey indicates that fertilizers product is not tested for heavy metals thus resulting in heavy metal build up in soil and subsequent soil organism poisoning. Thus the aim of this work was to investigate the heavy metals incidence in the application or inorganic and organic fertilizers to farm soil and the biochemical responses of earthworm Eisenia Fetida (Savigny) exposed to the treated fertilizer soils
2. Materials and Methods
2.1. Materials
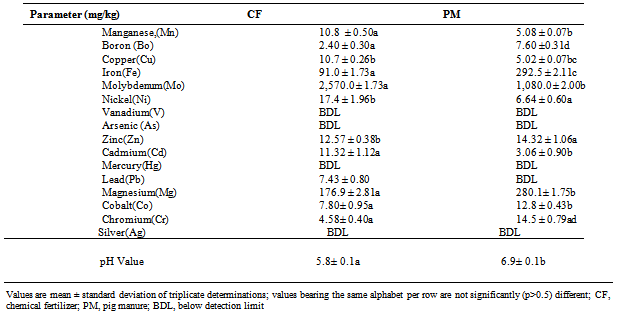
- The inorganic fertilizer (N15P15K15 products of Federal and Chemical Company Limited Kaduna) was purchased in full bag from a market in Aba metropolis while the organic manure (composted pig manure) was collected from pig farm located in Aba North Local Government Area of Abia State, Nigeria. N15P15K15, and pig manure were denoted as CF and PM respectively. All chemicals and reagents used were from reliable sources and of high analytical grade. Heavy metal analyses were carried out using atomic absorption spectrophotometer (UNICAM 969, Japan) whiles the biochemical parameters were determined using spectrophotometer (model 390, Turner®, USA).
2.2. Soil Sampling
- Soil samples were collected from the different locations of the Abia State Polytechnic botanical garden Aba Abia State, Nigeria lying between latitude 4 .451 and longitude 30 and 80 91 East. The soil samples were collected by sterile method using plastic auger from a depth of 0-15cm[7]. The auger was washed with water and 70% ethanol before use.
2.3. Experimental Design
- The experiment included three treatments with three replication arrangements. The treatments were (a) no application of fertilizer (NF); (b) mineral NPK fertilizer application (CF); and (c) Pig manure application (PM).Each treatment had 0.0, 0.10 and 0.5g/kg soil fertilizer application rates. Fertilizers were fully mixed with soil on a plastic sheet and loaded into various sets of the experimental pots and were allowed for a latent period of 7 days. The moisture content of the soil was adjusted daily with 5-10ml distilled water to compensate for the lost water. The experimental pots were perforated with numerous pin-size holes at the bottom. The adoption of this mild leaching method was done to reflect the true environmental solution. The sets of earthworms were exposed to the various fertilizer application rates and allowed for a period of 28 days in the laboratory.
2.4. Soil and Fertilizer Analyses
- The soil and fertilizer samples were anlaysed using the method of the American Standard Testing Material[8] as described in[6]. The samples (2.0g) were weighted into a beaker and digested with 20ml of conc. HNO3 and 10ml of conc. HClO4 on a hot plate with gentle boiling. The digested samples were evaporated to dryness and the residue mixed with 10ml of 2.0 M HCl, filtered into a 100ml standard flask using whatman No1 filter paper. Several digested samples were analyzed for the heavy metal of interest – Mn, B, Cu, Fe, Mo, Ni, Zn, Mg, V, As, Cd, Hg, Pb, Co, Cr and Ag using atomic absorption spectrophotometer (Unicam 969,Japan). The samples were analyzed in triplicates. The effectiveness of the method used was tested by using spiked samples that were later used as reference samples. Soil particle fractionation and moisture content were carried out according to the methods of[8]. The pH was determined using glass electrode pH meter at the ratio of 1:2 sample / water suspensions[9].
2.5. Biochemical Assay
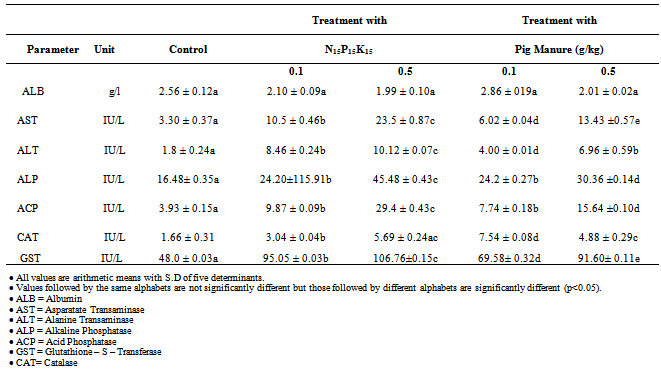
- The biochemical parameters were done on individual earthworm at a dilution ratio of 1:2w/v using Tris buffer. Each earthworm was rinsed with distilled and deionized water (< 20 seconds) and was cut and crushed using a laboratory mortar. The resulting crushed tissues were centrifuged at 7500r/min for 15min and the supernatant was removed and stored in a refrigerator at 4oC and biochemical assays, done within 7 days[9].Coelomic fluids were tested for aspartateaminotransferase (AST, EC.2.6.1.1), and alanine aminotransferase (ALT, EC.2.6.1.2), according to[10]. This method is based on the action of the aminotransferases on their substrates to liberate their corresponding amino acids, which react with 2.4 dinitrophenylhydrazine (DNPH) to form coloured complex. Consequently, enzymes activities are measured in relation to the concentration of the colour solutions. Each clarified sample (0.1 ml) was added into a clean test-tube containing 1ml of the corresponding substrate. The mixture was incubated at 37℃ in a waterbath for 15mins and then 0.5ml of DNPH and 0.5ml 0.4N sodium hydroxide (NaOH) were added. The absorbance of the samples and the standard (sodium pyruvate) were read in the spectrophotometer against the reagent blank. The alkaline phosphatase (ALP, EC.3.1.3.1) and acid phosphatase (ACP, EC.3.1.3.2) were estimated according to[11]. This is based on the action of the phosphatases on p-nitrophenylphosphate to liberate p-nitrophenol and organic phosphate, which at acid/alkaline pH values changes colour that can be spectrophotometrically determined. Each clarified sample (0.1ml) was added a clean test tube containing 1ml of corresponding substrate. The mixture was incubated at 37℃ in a water bath for 15mins and then 0.8ml of 0.5N NaOH, 1.2ml of 0.5N sodiumhydrogentrioxocarbonate(IV) (NaHCO3), 1ml of 4-amino-1 phenyl-2,3 methyl-5-pirazolon, and 1ml of potassium ferricyanide were added. The absorbance of the yellow coloured solution produced and the standard (n-nitrophenyl solution) were read in the spectrophotometer against the reagent blank. Catalase (CAT, EC.1.11.1.6) was measured using the Northwest life science specialties kit USA (NWLSSTM). Catalase reduces the hydrogen peroxide which can induce damage in the membrane by initiating lipid peroxidation[1]. A portion of the supernatant (0.1ml) was added to cuvette containing 1.0ml of assay buffer, 1.0ml of sample dilution buffer and 1.0ml of H2O2 regent. The absorbance of the solution was read immediately in a spectrophotometer. The rate of disappearance of H2O2 was followed by observing the rate of decrease in the absorbance and latalase activity calculated.Glutathione-S-transferase (GST, EC.2.5.1.18) were measured by the method of[1]. A portion of the supernatant (0.1M) was added to curvette containing 2.75ml of sodium phosphate buffer (0.1m pH 7.0), 0.10ml reduced glutathione (1.0mM) and 0.05nm 1-chloro-2,4-dinitrobenzene making the total volume 3.0ml. The mixture was quickly mixed and the absorbance read at 340nm. The blank underwent the same treatment as the sample except that distilled water was used in place of sample. The values obtained were subtracted from the blank to give the catalytic activity of GST.Coelomic fluid albumin concentration was determined using bromocresol green method as described by[12]. The samples and indicator (bromocresol green) were pipette into labeled test tubes as indicated in the procedure adapted from[12]. When the indicator binds to albumin, the colour changes and the absorbance of the colour produced was measured using spectrophotometer.
3. Environmental Impact Assessment
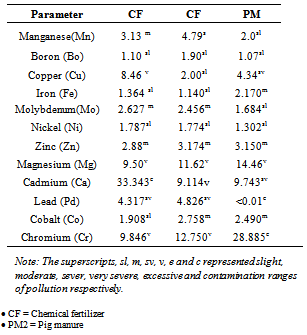
- The pollution index per metal with a significant quantity considered from pi> 1 was used as assess the effect of the control soil as described by[13]. Pi is a measure of a metal concentration in fertilizer treated soil samples and its concentration in a background sample. Statistical analysis is based on the following mathematical relations.Pi = Cm/CbWhere Cm is the concentration of the metal obtained in the fertilizer – treated soils and Cb is the relative background of concentration of the metal. Pollution range was classified into intervals that define a slight (1.1-2.0), moderate (2.1-4.0), several (4.1-8.0) very severe (8.1-16.0) and excessive pollution (>16.0) respectively.
4. Results and Discussion
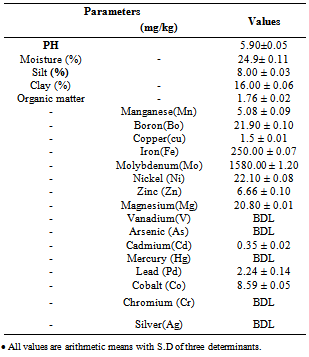
- Textural analysis showed a preponderance of sand fraction (76.00%) followed by clay (16.00%), then silt (8.00%) thus classifying the soil as sandy clay as presented in Table 1. Although sandy soils are known to have a poor retention capacity for water and metals, the relatively moderate proportion of clay suggests that the soil will not drain easily with implications for potential deleterious impact of retained pollutants on environmental receptors. The acidic pH 5.90±0.05 recorded for the soil is within the range for agriculture soil[14]. Soil pH plays a major function in the sorption of heavy metals as it controls the solubility and hydrolysis of metal hydroxides. The soil also showed moderate organic matter content of 1.75± 0.02%.
|
|
|
|
5. Conclusions
- These results showed that heavy metals could interfere with the normal biochemical metabolic processes by the generation of free radicals. The responses were particularly sensitive as significant changes were detected at the lower concentration of 0.1g/kg application rate. The research indicated that AST, ALT, ALP, ACP, CAT and GST can be used as biomarkers of cellular damage, AST, ALP and GST showed more sensitivity with significant difference (p<0.05) in concentrations than ALT, ACP and CAT respectively. These biochemical responses measured in E. fetida (Savigy) could be used for chemical testing in the laboratory or for soil contamination surveys.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the technical assistance received from the staff of Intentional Energy Services Limited Port-Harcourt, River State. Nigeria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML