-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(3): 67-73
doi:10.5923/j.ajb.20130303.01
Phytochemical and Heavy Metal Composition of Telfairia Occidential and Talinium Triangulare Grown in Aba Nigeria and Environmental Health Implications
Ukpabi Chibueze1, Akubugwo Emmanuel I.2, Agbafor Kingsley3, Wogu Chidi4, Chukwu Henry C.4
1Department of Biochemistry, Abia State Polytechnic Aba
2Department of Biochemistry, Abia State University Uturu
3Department of Biochemistry, Ebonyi State University Abakaliki
4Department of Chemistry, Abia state Polytechnic Aba
Correspondence to: Ukpabi Chibueze, Department of Biochemistry, Abia State Polytechnic Aba.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Phytochemical and heavy metal composition of Talinum triangulare and Telfairia occidentalis grown in Abia State Polytechnic garden in Aba, Nigeria and the environmental health implications were determined, where inorganic fertilizers are used for cultivating vegetables. The experimental garden was divided into two portions, control and the treated portion. The heavy metal composition of the topsoil of the garden and the plant species were studied using Atomic Absorption Spectrophotometry (AAS). Transfer Factor (TF) and Percentage Metal Uptake (PMU) were the key parameters used to assess human exposure to heavy metal through the food chain. Phytochemical studies indicated alkaloids, saponins, phenols and flavanoids in the plant samples while tannins were only dictated in T. triangulare. Though there was an increase in saponins and flavanoids grown in the fertilizer treated portion of the garden, they were not significant (p>0.05) when compare to the control sample. The heavy metal analysis in the soil and vegetable samples indicated the presence of Iron (Fe), Zinc(Zn), Copper(Cu), Nickel(Ni), Cadmium (Cd), Lead (Pb), Molybdenum (Mo), Cobalt (Co) and Chromium (Cr) while Vanadium(V), Arsenic(As), Mercury(Hg) and Silver(Ag) were not detected. Among the heavy metals studied Ni was the most mobile within the plant while Cr was the least mobile. T. triangulare accumulated relatively greater amount of Cd and Pd and also translocated the greater amount of Cd to its leaves than Telfairia Occidentalis, hence it may be less risky to eat Telfairia occidentalis than Talinum triangulare. The daily intake of these metals at present may be less than theoretical concentrations that affect health, the situation could however change in the future depending on the dietary pattern and the volume of metal pollutants added to the ecosystem.
Keywords: Phytochemical Composition, Heavy Metal Content, Talinum Triangulare Telfairia Occidentalis, Health Risk
Cite this paper: Ukpabi Chibueze, Akubugwo Emmanuel I., Agbafor Kingsley, Wogu Chidi, Chukwu Henry C., Phytochemical and Heavy Metal Composition of Telfairia Occidential and Talinium Triangulare Grown in Aba Nigeria and Environmental Health Implications, American Journal of Biochemistry, Vol. 3 No. 3, 2013, pp. 67-73. doi: 10.5923/j.ajb.20130303.01.
Article Outline
1. Introduction
- Vegetables constitute important part of the human diet, since they contain carbohydrates, proteins, fats, as well as vitamins, minerals and bioactive compounds[1].in recent years, consumers demand for better green leafy vegetables is rapidly increasing among the urban community[2]. This is due to increased awareness on their nutritive and herbal values[3]. These vegetables are harvested at all stages of growth and fed either as processed, semi-processed or fresh to man while they are usually offered fresh to livestock[4]. The perceptions of what is regarded as a better quality are however subjective. Some consumers consider colour and size as characteristics of good quality leafy vegetables. However, physical characteristics of leafy vegetables alone cannot guarantee safety from heavy metal contamination.Leafy vegetables have greater potential of accumulating heavy metals in their edible parts than grain or fruit crops because they are largely xylem-loaded and heavy metals are generally greatly mobile in the xylem[5];[6]. Contamination of human food chain by heavy metals is not directly affected by the plants total uptake but rather by the concentration in their parts that are directly consumed[7]. According to Lee et al.,[8] sensitivity of animals to heavy metals toxicity depends on heavy metal accumulation rate in the edible parts of the plants and intake rate, amongst other factors. Heavy metals rank high amount the major contaminants of leaf vegetables[9]. Heavy metals become toxic when they are not metabolized by the body accumulate in the soft tissues[10]. Recent studies have revealed that those who cannot excrete heavy metals efficiently appear to be genetically predisposed to this condition[11]. Several studies have shown that the presence of toxic heavy metals in biological systems can cause oxidative stress which promotes the formation of reactive oxygen species that damage cells[12]. Plants scavenge and dispose of these reactive molecules by use of antioxidant and defense system present in several sub-cellular components. The major antioxidant species in plant are ascorbate, reduced glutathione,
 -tocophenol and carotenoids; polyamines and flavonoids also may provide some protection from free radical injury [13];[14]. The medical significance of oxidative stress has become increasingly recognized to the point that it is now considered to be a component of virtually every disease process including aging, coronary heart diseases, inflammation stroke, diabetes mellitus and cancer[15]. The beneficial health effects of vegetables have been attributed in part, to antioxidant phytochemical compounds present in them[16];[17] and their ability to chelate some heavy metals and prevent their absorption from ingested food.In Nigeria, inorganic and organic fertilizers are widely used and have been implicated as the chief source of heavy metal pollution of agricultural fields[10]. The production of leafy vegetables using mechanized agricultural techniques is well practiced in Abia State of Nigeria but little is known on the contamination of leafy vegetables with heavy metals. Talinum triangulare and Telfairia Occidentalis are some of the commonly eaten edible leafy vegetable species in Nigeria and are consumed all through the year[18]. The main objectives of this study were to determine the phytochemical composition and the accumulation of some heavy metals in the leaf tissues of Talinum triangulare and Telfairia Occidentalis grown in Abia State Polytechnic Garden, Aba and to estimate their environmental health risks.
-tocophenol and carotenoids; polyamines and flavonoids also may provide some protection from free radical injury [13];[14]. The medical significance of oxidative stress has become increasingly recognized to the point that it is now considered to be a component of virtually every disease process including aging, coronary heart diseases, inflammation stroke, diabetes mellitus and cancer[15]. The beneficial health effects of vegetables have been attributed in part, to antioxidant phytochemical compounds present in them[16];[17] and their ability to chelate some heavy metals and prevent their absorption from ingested food.In Nigeria, inorganic and organic fertilizers are widely used and have been implicated as the chief source of heavy metal pollution of agricultural fields[10]. The production of leafy vegetables using mechanized agricultural techniques is well practiced in Abia State of Nigeria but little is known on the contamination of leafy vegetables with heavy metals. Talinum triangulare and Telfairia Occidentalis are some of the commonly eaten edible leafy vegetable species in Nigeria and are consumed all through the year[18]. The main objectives of this study were to determine the phytochemical composition and the accumulation of some heavy metals in the leaf tissues of Talinum triangulare and Telfairia Occidentalis grown in Abia State Polytechnic Garden, Aba and to estimate their environmental health risks. 2. Materials and Method
2.1. Study Area
- Vegetable garden at the Abia State Polytechnic Aba was used for the study. The garden has been under cultivation for at least fifteen years. Abia State Polytechnic is situated in Aba, Abia State Nigeria. Abia State is created out of Imo State in August 27, 1991. The state is located in the south eastern part of Nigeria and it lies between latitude 4o 45 and 6 o 15 North and longitude 6 o30 and 8 o 9 east. It is bordered on the North and north east by Anambra and Enugu States respectively and on the east by Cross River and Akwa Ibom States. Its southern border is shared with River State.There are two diametric seasons: the dry season and the wet season. The wet season generally commences in April through September. The peak of the rainfall occurs in July with a short slightly drier period called the “August break”. The mean annual rainfall is between 1800 and 2500mm per year. The maximum and minimum temperatures are 31.90℃ and 22℃ respectively while the daily sunshine rate is about 4.4 hours. The dry season spans from October to March.
2.2. Sampling
- Seeds of the two vegetables namely Telfaria occidentalis (fluted pumpkin) and Talinum triagulare (water leaf) were collected from the Ministry of Agriculture Aba, Abia State and the Voucher specimen of the seeds were deposited in Herbarium, Department of Plant Science and Biotechnology, Ebonyi State University, Abakiliki, after proper identification. The seeds of Telfaria occidentalis (fluted pumpkin) and Talinum triagulare (water leaf) were planted and allow to grow for a period of 12 weeks between the months of October to December, 2011.
2.3. Preparation of Plant Samples
- Vegetable leaf and root samples were collected by random picking and were washed with distilled and deionized water. The samples were sun dried and ground into uniform power using an electric machine blender and then stored in an air-tight bottle till required for analysis.
2.4. Phytochemical Analysis
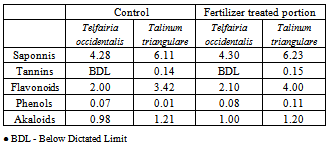
- One gram powder of each of the plant samples was subjected to qualitative phytochemical tests for alkaloids (wagner dragendroff’s reagent), saponins (chloroform and H2SO4 tests), tannins (ferric salt tests), flavonoids (ammonium test) and glycosides(ferric chloride and H2SO4 tests) as adopted by Harborne[19] Quantitative tests for the determination of the phytochemical constituents of the plant samples were done according to Harborne[19] as described in Ukpabi, et al.,[20]
2.4.1. Alkaloids Content
- This was done by the alkaline precipitation gravimetric method. Each of the samples (5g) was weighed into a 250ml beaker and 200ml of 20% acetic acid in ethanol (1:10) was added and covered to stand for 4hrs. This was filtered and the extract was concentrated using water –bath to one-quarter of its original volume by evaporation and treated with drop-wise addition of conc. aqueous NH4OH until alkaloids was precipitated. The whole solution was allowed to settle and the precipitate was collected by filtration which was later dried and weighed. The alkaloid content was calculated and expressed as mg/100g of the weight samples analyzed.
2.4.2. Tannins Content
- Tannin content was determined by the photometric method. The samples (5g) were weighed into 100ml plastic bottle. 50ml of distilled water was added and shaken for 1hr in a mechanical shaker. This was filtered into a 50ml volumetric flask and made up to the mark with distilled water. Then 5ml of the filtrate was pipette out into a tube and mixed with 3ml of 0.1M FeCl3 in 0.1N HCl and 0.008M potassium ferrocyanide. The absorbance was measured in a spectrophotometer at 760nm wavelengths, within 10 minutes. A blank sample was prepared using the extraction solution (distilled water). A standard solution of tannic acid (5mg/ml) was prepared and with the blank reagent at zero the standard absorbance was measure at 760mm wavelength.
2.4.3. Saponins Content
- Each of the plant samples (5g) were dispersed in 200ml of 20% ethanol. The suspension was heated over a hot water bath for 4hrs with continuous stirring at about 55℃. The mixture was filtered and the residue re-extracted with another 200ml of 20% ethanol. The combined extracts were reduced to 40ml over a water bath at about 90℃. The concentration was transferred into a 250ml separator funnel and 20ml of diethyl ether was added and shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated after which n-butanol (60ml) was subsequently added and the combined n-butanol extracts were washed twice with 10ml of 5% aqueous sodium chloride. The remaining solution was poured into a dried pre-weighed crucible and dried in an oven at 60℃ to a constant weight.
2.4.4. Flavonoids Content
- Each of the plant samples (5g) was weighted into 100ml plastic bottle and extracted repeatedly with 100ml of 80% aqueous ethanol at room temperature. It was filtered with Whatman No 42 fitter paper into 100ml flask. The filtrate was transferred into a crucible dish and evaporated to dryness over a water bath. This was further dried in an oven at 60℃ at 30 minutes and cooled in desiccators. The crucible and the content were weighted and recorded.
2.4.5. Total Phenolic Content
- For the extraction of the phenolic component, the fat free sample was boiled with 50ml of ether for 15 minutes. The extract (5ml) was pipette into a 50ml flask, and 10ml of distilled water was added. 2ml of ammonium hydroxide solution and 5ml of concentration amyl alcohol were also added. The samples were left to react for 30 minutes for colour development. The absorbance of the solution was read using a spectrophotometer at 505nm wavelength. A blank sample for each extract was used for background subtraction. A standard phenol was prepared as 0.05mg/l and absorbance measured. The total phenolic content was expressed as mg/100g.
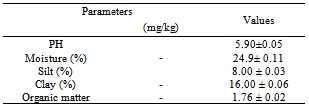
2.5. Soil Physical Analyses
- Soil particle fractionation, moisture content and pH were carried out according to the method of Nwinuka et al.,[21], while organic matter was done as described by Lu[22].
2.5.1. Soil Particle Fractionation
- Oven-dried sieved soil sample (50g) was weighed and put into a polythene bottle. Sodium hexametaphosphate solution (5%, 25ml) used as dispersing agent was also added. Distilled water (400ml) was subsequently added and the suspension was thoroughly mixed together for 2 hours. The suspension was quantitatively transferred to a litre cylinder and then made up to the 1000ml mark. The suspension was stirred with a glass rod and then measured using soil hydrometer. The readings were taken in triplicates. Silt and clay readings were taken at 4 minutes while clay readings were taken at 5 hours. The temperature of the suspension was taken after each reading.
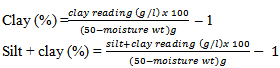 Where 1 is the dispersing agent correlation factor.% silt = (silt + clay) (%) – clay (%)Total sand = 100 – (silt + clay) (%)
Where 1 is the dispersing agent correlation factor.% silt = (silt + clay) (%) – clay (%)Total sand = 100 – (silt + clay) (%)2.5.2. Moisture Content
- Fresh soil sample (50g) was weighed and put into a known weight crucible. The sample was placed in an oven at 105oC and dried. It was cooled and re weighed. The percentage moisture from loss in weight was calculated as
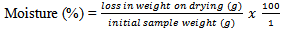 These experiments were repeated in triplicates.
These experiments were repeated in triplicates.2.5.3. Soil pH
- Air-dried soil sample (10g) was weighed and put into a 50ml beaker. 20ml of deionized water was added and allowed to stand for 30 minutes. The mixture was stirred occasionally with a glass rod and the pH measured by inserting the electrode of the pH meter into the suspension after standing for 15 minutes. The result was reported as “soil pH measured in water”. The pH meter was checked and adjusted before the measurement of the suspension using two buffer solutions, buffer solution I on one side of the meter range and buffer solution II on the other side. The pH of the soil sample was measured in triplicates and the average result recorded.
2.5.4. Soil Organic Matter
- Air-dried, crushed and sieved soil sample (20g) was weighed and transferred into a 250ml of round bottom flask. 10ml of 0.25N Potassium dichromate VI (K2 C12 O7) solution was pipetted into the flask and then swirled gently to disperse the soil. 20ml of concentrated tetraoxosulphate (VI) acid (H2SO4) was rapidly added using an glass pipette. The flask was swirled until soil and reagents were mixed and were allowed to stand on a sheet for 30 minutes. 3 drops of indicator (Ferroin indicator) was added and then titrated with 0.1N standard ammonium iron (ii) sulphate solution (NH4)2Fe(SO4)2. The ammonium iron (ii) sulphate was added drop by drop until the colour changed sharply from blue to red. The blank was run along side the samples. The quality of oxidazable organic matter in the soil sample was estimated in terms of oxidizing equivalents.
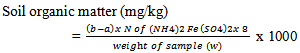 Where b = ml (Volume) of the titrant with blanka = ml (volume) of the titrant with sampleN = Normality of ferrous sulphateW = weight of the sample used.
Where b = ml (Volume) of the titrant with blanka = ml (volume) of the titrant with sampleN = Normality of ferrous sulphateW = weight of the sample used.2.6. Heavy Metal Contents in Soil and Plant Materials
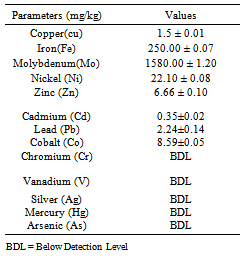
- Atomic absorption spectrophotometric (AAS) method as described in Ukpabi et al[10] in determination of heavy metals in plant and soil samples. Each of the sample (2g) was weighed into the beaker and digested with 20ml of conc. HNO3 and 10ml of conc. HCLO4 on a hot plate with gentle boiling. The digested samples were evaporated to dryness and the residue mixed with 10ml of 2.0M HCL filtered into a 100ml standard flask using Whatmann No 1filter paper. several digested samples were analysed for the heavy metals of interest Mn, B, Cu, Fe, Mo, Ni, Zn, Mg, Ca, V, As, Cd, Hg, Pb, Co, Cr, Ag using an atomic adsorption spectrophotometer(UNICAM 969 Japan). The effectiveness of the methods used was tested by using spiked samples that were later used as reference samples.
2.7. Environmental Impact Assessment
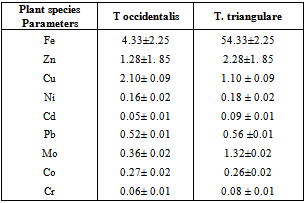
- In this study, two different criteria were used for the environmental impact assessment.Transfer factor per metal (TF) is a common parameter often used in the study of the environmental contamination[23]. TF is the ratio of concentration of metal in aerial plant part and the underlying soil.TF=Cp/CbWhere Cp is the concentration of the metal obtained in the aerial part of the plant Cb is the relative background concentration of the metal in soil.Percentage metal uptake is the roots to leaves movement of the elements. The leaves to roots concentration ratio of metals give the compositions of the plant with regard to the percentage metal from the soil[24].
 M= Concentration of metals.
M= Concentration of metals.3. Result and Discussion
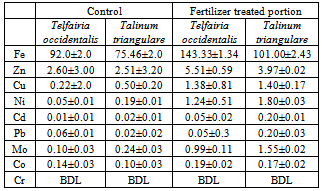
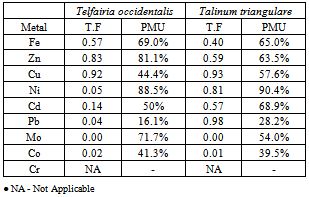
- The variation of the heavy metal content, of the topsoil (0-15cm) of the garden was reported in the Table 2. The result revealed Mo (1580.00± 1.20mg/kg) as the highest value while V, As, Hg Cr, and Ag were not detected by our analytical method. The concentrations (mg/kg) of the other detected metals followed the sequence Fe > Ni > Co > Zn > Mn > Pb > Cu > Cd. The garden soil showed heavy metal contents within the ranges of threshold values reported in literature for agricultural soils[25], thus established the suitability of the soil for planting. The Mo concentration observed in the garden topsoil may suggest that the garden had been managed with inorganic fertilizer previously[10].Heavy metal concentrations in the leaf and root tissues of T.occidentalis and T.triangulare grown at the garden were shown in Table 3 and 6 respectively. The values obtained in the plant species did not exceed the critical concentrations for plant growth and animal feed as reported by[14] Adverse health effect consequent upon comsumption of contaminated leafy vegetables has also received much attention, especially metals that are not essential nutrients for plants and animals. However, these metals at low concentrations can become toxic and constitute serious health problem, whenever they enter the human food chain. Recent research also revealed that people who cannot excrete heavy metal efficiently appeared to genetically predispose to this condition[11]. Telfairia Occidentalis accumulated more Fe in its leaf and root tissues than the leaf and root tissues of Talinium Triangulare. This could account for the therapeutic relevance of Telfairia Occidentalis in the treatment of anaemia as reported by[18]. The Fe concentrations were below the 425.00mg/kg value as recommended maximum level for vegetables[27]. Similar trend was observed when Zn concentrations were determined. T.occidentalis and T.trianugulare recorded Zn (5.51± 0.59 and 3.97±0.02 µg/g) as the second value among other metals as this element is known to be a mobile metal. Mapandu et al.,[9] indicated that Zn phytotoxicity in most leaf vegetables occurs when Zn accumulated to an average tissues concentration of 500mg/kg. This is far from the concentrations of Zn found in these vegetables. Cu, Ni, Mo and Co recorded their higher values in the leaf and root tissues grown in the chemical fertilizer treated portion of the garden than the control. The concentrations of Cu, Ni, Mo and Co in the leaf and root tissues of Talinium Triangulare where higher than the concentrations in the leaf and root tissues of Telfairia Occidentalis. This could indicate that Talinium Triangulare may have phytoremediation qualities to extract heavy metals from the soil than Telfairia Occidentalis. The concentrations of Cu, Ni, Mo and Co in the T.occidentalis and T.trianugulare leaf and root tissues at the garden site were below the FAO/WHO recommended limits (20-100µg/g dry wt). In the same vein, Cd and Pb recorded higher values in the leaf and root tissues of Telfairia Occidentalis and Talinium Triangulare grown in the inorganic fertilizer treated portion of the garden than the control. Cd and Pb also recorded higher values in the leaf and root tissues of Talinium Triangulare than in Telfairia Occidentalis. These observations agree well with[30] that toxic heavy metals introduced to the soil after phosphate fertilizer applications were accumulated by edible vegetables. Telfairia Occidentalis was generally the fewer accumulators of Cd and Pb comparing to Talinium Triangulare in this experiment.Transfer factor and percentage metal uptake were the basic parameters used to assess human exposure to heavy metal through the food chain[3]. The parameters quantify the relative differences in bioavailability of soil metal to plant and translocation within plant, which is the functions of both soil and plant properties. For instance the transfer factor values of the detected metals followed the sequence: Cu> Zn> Fe> Cd> Ni> Pb> Co>Mo and Cu> Zn> Cd> Fe> Pb> Ni> Co>Mo for Telfairia Occidentalis and Talinium Triangulare respectively.Cu and Zn which are common micronutrients for the plants were found as mostly absorbed in the leaf and root tissues of the vegetables. The quantity absorbed by the plants from the treated portion was higher, though their distribution was not uniform in the two plants species. The percentage metal uptake of the metals followed the sequence: Ni> Zn> Mo> Fe> Cd> Cu> Co> Pb and Ni> Cd> Fe> Zn> Cu> Mo> Co> Pb for Telfairia occidentalis and Talinium triangulare respectively. Among the heavy metals studied, Ni was the most mobile within the plant while Cr was the least accumulated in the leaf and root tissues. Similar trends were also observed in Kabata-Pendias and Pendias, 1992 and Ayari et al., 2010. This showed that Cu and Zn uptake from the soil to the roots of the plant species was much more efficient than their translocation from the roots to the leaves. Contrarily, Ni was efficiently translocated from the roots to the leaves than its uptake from the soil to the roots. Mo recorded the highest value in the top soil of the garden but showed low values of transfer factor and percentage metal uptake. Although the values of the percentage metal uptake were relatively higher than the values of the transfer factor.One of the major findings in this study is that the accumulation of the non-essential/toxic heavy metals varied among the plant species examined. For example, Talinium triangulare accumulated relatively greater amount of Cd and Pb in the roots and also translocated the bigger amount of Cd to its leaves than Telfairia occidentalis. Relatively, the uptake of Cd by the two vegetables was greater than the uptake of Pb. This observation is not surprising since tight binding of Pb to soils and plant material, are at least partially explains the relatively low mobility of this metal in soils and plant[27]. Pb binding to clay and organic matters and its inclusion in insoluble precipitates makes significant fraction of Pb unavailable for root uptake[3]. Again the uptake mechanism of the non-essential toxic metal has been show to be due to competition with respect at absorption of essential metal[31]. Non-essential metal may effectively compete for the same transmembrance carrier used by essential heavy metal. The earlier work of[12] and[27] have intrigued the possible detrimental links between micronutrient transport and human health, given that some essential micronutriens also can be classified as toxic heavy metals (eg, Zn, Cu and Ni) or are analogs of heavy metal (eg Zn which is an analogs of Cd). This relative lack of selectivity in transmembrance ion transport may partially explain why non-essential heavy metals can enter cells, even against a concentration gradient.The practice of growing vegetables especially those ones that have beneficial medicinal effects round the year are rapidly increasing among the urban communities. The practice may not be safe and sustainable in long term if abused especially with the use of inorganic fertilizers. However the vegetables at the site under this study could be contributing insignificantly to Cd and Pb intake. This depends also on the dietary p attern of the consumers. The average amount of vegetable consumed per day by a person in most of the developing countries in Africa is less than the international daily coverage of 50g for leafy vegetable[2]. It is because of this that the intake of heavy metals from plant food constitution much less than the theoretical maximum daily intake which are used to express the exposure of consumers and associated health risks. Children are more susceptible to adverse effects of Cd and Pb because they eat more food relative to their body mass than adults. They absorb Cd and Pb more readily than adults and their major organs, including the brain, are still under developed making them the most susceptible.
|
|
|
4. Conclusions
- Based on the result of this study T. occidentalis and T. triangulare have been shown to contain phytochemical compounds and accumulate heavy metals. Potential public health hazards, especially from Cd and Pb are low at this site.
5. Recommendations
- The practice of growing vegetables using fertilizers especially inorganic fertilizers is aimed at increasing the agricultural yield. However, there is need for an improved food quality assurance system and evaluation of total dietary consumption rate for urban and rural dwellers in order to assess human toxicity.
ACKOWLEDGMENTS
- The authors wish to acknowledge the technical assistance received from the staff of International Energy Services Limited port-Harcourt, River State and Yamac Consulting and Analytical Services Limited Aba, Nigeria.
Appendix
|
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML