-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(2): 29-33
doi:10.5923/j.ajb.20130302.02
Carbaryl Induced Changes in the Protein and Cholesterol Contents in the Liver and Muscle of Marine Benthic Fish, Mugil cephalus
Shivanagouda. N. Sanagoudra, U. G. Bhat
Department of Studies and Research in Marine Biology, Karnataka University, P. G. Centre, Karwar, Kodibag, Karnataka, 581 303, India
Correspondence to: Shivanagouda. N. Sanagoudra, Department of Studies and Research in Marine Biology, Karnataka University, P. G. Centre, Karwar, Kodibag, Karnataka, 581 303, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The effect of carbaryl insecticide was tested invivo on fresh water fish Mugilcephalus. Toxicity tests were conducted to measure the impact of toxicity on the protein and cholesterol levels in liver and muscle of fish. During our present investigation fishes were exposed to the lethal and sub lethal concentration of carbaryl for 4 days 21 days respectively. The levels of protein and cholesterol were determined by standard procedures in the liver and muscle tissues of unexposed (control) fish and the fish exposed to carbaryl. The results showed significant fall in the cholestrol and protein content of liver and muscle on carbaryl exposure. The fluctuations in these are indicative of the organism’s response to the toxicant stress.
Keywords: Carbaryl toxicity, Protein, Cholesterol, Liver, Muscle, Mugilcephalus
Cite this paper: Shivanagouda. N. Sanagoudra, U. G. Bhat, Carbaryl Induced Changes in the Protein and Cholesterol Contents in the Liver and Muscle of Marine Benthic Fish, Mugil cephalus, American Journal of Biochemistry, Vol. 3 No. 2, 2013, pp. 29-33. doi: 10.5923/j.ajb.20130302.02.
Article Outline
1. Introduction
- Toxicology is the measurable study of the deleterious effects due to chemical or physical agents, which cause adverse effects of chemical on living organisms. The commonest toxic agents in India appear to be chemical pesticides, sedative drugs, chemicals, alcohol, plant toxins and household poisons. Carbamate insecticides are derivatives of carbamic acid, HOC (O) NH2. Carbamates are gradually replacing organophosphates which are more toxic and persistent as widely used insecticides, herbicides, fungicides, rodenticides etc, in agriculture and households. The carbamate pesticides are biodegradable with the low mammalian toxicity, have wide spectrum of activity, are quick acting and more economic. They therefore occupy a prominent place in eradicating various pests of food crops in agriculture.Combined toxic effects of carbaryl and methyl-parathion were investigated on survival, growth and respiratory metabolism in Heteropneustes fossillis[1]. They demonstrated that methyl parathion was more toxic than carbaryl in individual concentration and in combination produced synergistic and antagonistic effects. Disease, starvation, aging, stress and environmental factors are known to influence physiological and biochemical state of animals by exhibiting marked changes in the activities of several enzymes[2];[27];[29]. Changes in enzymes of Carbohydrate metabolism under pesticide impact are well documented[3][7]. Effects of methomyl pesticide on Protein levels in tissues of Oreochromis mossambicus was studied[8]. Chronic poisoning of fish with organophosphate and carbamate compounds leads to profound changes in the Protein metabolism[9]. The pesticides are also known to inhibit energy synthesis by suppressing aerobic oxidation of Protein leading to energy crisis in animals[10].The present approach consists of evaluating Protein and cholesterol levels in tissues of Mugil cephalus under lethal and sublethal concentrations of carbaryl.Although a considerable work has been carried out to study the chemical toxicity effects on biochemical changes of organism, still much work needs to be done. Furthermore, it is essential that the commercial forms as well as their subsequent changed formulations to be tested on an organism. Since, the activity of a pesticide may be markedly altered by the formulation factors employed[11]. Hence, it is with this background the formulation of carbamate namely; Sevin was evaluated for their toxic affects in common carp, Mugil cephalus.
2. Materials and Methods
- Mugil cephalus fingerlings (Average length 8-15cm; Average weight 4-12g) were obtained from Central Institute of Fisheries Research Education Mumbai, Aquaculture and Fishery resource Management Division, and carried in oxygenated polythene bags within an hour to the laboratory. The fishes were acclimatized in the laboratory for two weeks in large plastic troughs. During acclimation, the fishes were fed with pellets of aller aqua feed containing 37% protein and 12% lipids. Water in trough was changed twice daily. Feeding was stopped a day prior to the commencement of the experiment.The carbaryl solution was prepared by usingcommercially available carbaryl 50% effective concentration. Stock solution of mg/l concentration was prepared. For working concentration the required dilutions were made in water.In order to determine the LC50, 10 yearlings of almost equal size, were released in each trough series containing test solution of concentrations of 4.0, 5.0, 6.0, 7.0, and 8.0 mg/l along with control. Their mortality was recorded at the end of 24, 48, 72 and 96 h. The LC50 value of 96 hr was considered as the lethal concentration, whereas, one third of it was taken as sublethal concentration.After exposing the fishes to the lethal and sublethal concentrations for 4 days and 21 days separately, the protein and cholesterol levels in liver and muscle were estimated by standard procedures[12. Liver tissue was homogenized in 0.9 mol/L NaCl (1:10 w/v) for the estimation of cholesterol, total protein, and liver tissue (0.25 g) was homogenized using 7% trichloroacetic acid for the extraction of adenosine nucleotides according to the method of Wijsman[28]. For estimation of PFK, 0.25 g liver was homogenized in 50mmolTrisHCL, 1mmoldisodium thylenediam-inetetra acetate dehydrate (EDTA) and 5mmol MgSO4 at pH 8.2 and then centrifuged for 10 min at 0 °C. The supernatant was used for enzyme assays while 0.25 g tissue was homogenized in 5 mL Tris-HCL buffer pH 7.6 for detection of protein. On the other hand, 0.25 g tissue was homogenized in 100mmol maleate-NaOH-buffer (pH 6.6) containing 20mmol NaF, 1mmol EDTA, 0.5 mg/mL bovine serum albumin and 10 mmol DL-dithothreitol for estimation of cholesterol.
3. Results
3.1. Protein
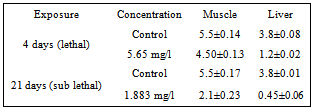
- The mean protein content of the muscle of control fish was 5.5 mg/g wet wt of the tissue, while in lethal concentration for 4 days it was 4.5mg/g wet wt of tissue, and 2.1 mg/g wet wt of tissue, in sublethal concentration after 21 days of exposure (Table 1). The Protein content of the liver of control fish was 3.8 mg/g wet wt of tissue, while in lethal concentration for 4 days it was reduced to 1.2 mg/g wet wt of tissue and 0.45 mg/g wet wt of tissue in sublethal concentration after 21 days of exposure. (Figure 1)
|
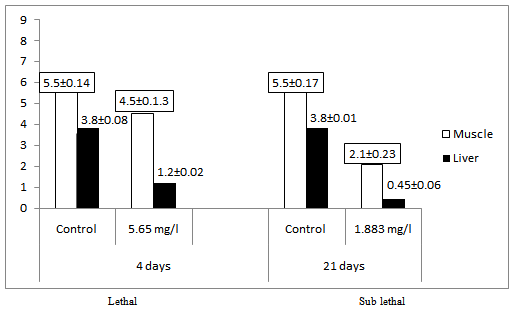 | Figure 1. Protein content (mg/g) of the tissue of the Mugil cephalus exposed to lethal (4 days) and sub lethal (21 days) concentration of carbaryl |
3.2. Cholestrol
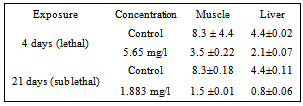
- The mean cholesterol content of the muscle of control fish was 8.3mg/g wet wt of tissue, while in lethal concentration for 4 days it was reduced to 3.5 mg/g wet wt of tissue and 1.5 mg/g wet wt of tissue in sublethal concentration after 21 days of exposure (Table 2). The cholestrol content of the liver of control fish was 4.4mg/g wet wt of tissue while in lethal concentration for 4 days it was reduced to 2.1 mg/g wet wt of tissue and 0.8 mg/g wet wt of tissue in sublethal concentration after 21 days of exposure (Figure 2).
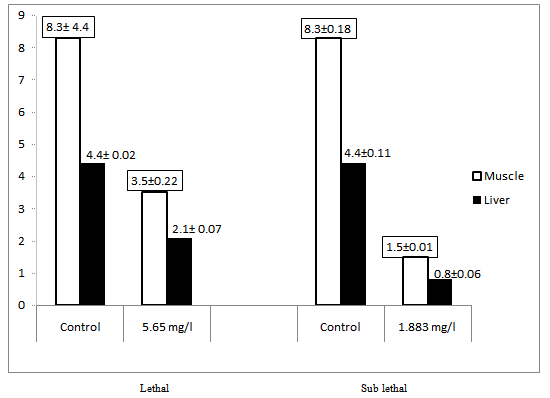 | Figure 2. Cholesterol content (mg/g) of the tissue of the Mugil cephalus exposed to lethal (4 days) and sub lethal (21 days) concentration carbaryl |
|
4. Discussion
- Pesticides in the aquatic environment cause several effects on the physiological and biochemical aspects of fish[13]. Biochemical changes in response to pesticides usually lead to irreversible and detrimental disturbances of integrated function such as behavior, digestion, growth, reproduction and survival itself, which in turn may cause many changes in the population level[14].Protein present the immediate and principal energy precursor for fish exposed to stress condition[15]. In the present study lethal and sub lethal concentration of carbaryl the cholesterol content in the liver and muscle reduced compared to control. Lowering in Protein level indicates energy requirement and energy mobilization. The excess energy requirement might be met through the mobilization of Protein and its significant alterations in blood-Protein, the proteins that have evolved mainly to participate in transport, also have permissive or secondary roles in mobilization of free fatty acids, state from 50 to 90 per cent of the body's total energy needs are met by free fatty acid has capabilities beyond the current requirements of many experimental, blood lactate and blood pyruvate level were observed in three species of fishes, i.e. Heteropneustes fossilis, Channa punctatus and Channa striatus abounding the polluted waters of Hussainsagar lake[30].There was decreased amount of cholestrol observed in pesticide exposed animals. It is reported that exposure to various toxicants considered stressful such as heavy metals, pulp mill effluents and pesticides[16];[17] causes reduction in the liver and muscle cholestrol of the fish. This is due to possibility of active cholestrololysis[18]. On methomyl pesticide exposure the carbohydrate level (cholestrol) was reduced in liver and muscle tissues of Oreochromis mossambicus over the control[1]. Toxic stress imposes an increased energy requirement from the animal to adapt to the changed metabolic conditions[19]. And this is achieved through the utilization of reserve store of Protein. In general, depleted levels of Proteins in fish tissue under toxic stress was explained as consequences of increased cholestrololysis. However reports are available showing an increased synthesis of catecholamine in fish during pesticide stress [20]. It may be due to acceleration of cholestrol tract down or inhibition of cholestrol synthesis as a result of pesticide interaction with endocrine system[21].It is an established fact that Protein can definitely be used as a source of energy[5];[22]. Proteins form an important constituent of animal tissues. It is one of the important macromolecules which comes first to reduce fish from endearing stresses caused by any Xenobiotics by providing energy for building material. A time dependent depletion of cholestrol content of liver and muscle tissue in Oreochromis mossambicus under the toxicity of the pesticide quinophos was reported[23].The observed depletion of both Protein and Cholesterol the present study explains the increased demand of these molecules to provide energy for the cellular biochemical process under toxic manifestation. Similar results were observed in Thalmile crenata, Anabas testudienues and Anabas scandens, when exposed tom copper, lead nitrate and mercury chloride respectively[24];[25]
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML