-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(2): 25-28
doi:10.5923/j.ajb.20130302.01
Production of Cellulolytic Enzymes by Aspergillus flavus Using Solid State Fermentation Based on Sugarcane Bagasse
Sideney Becker Onofre1, 2, Géssica C. Silva1, Shaiana P. Mattiello1, Deizi Groth1, Ivair Malagi1
1Department of Biological Sciences and Pharmacy, FAED - Faculty of Educational of Dois Vizinhos - União de Ensino do Sudoeste do Paraná – UNISEP - Av. Presidente Kennedy, 2601 –Bairro Nossa Senhora Aparecida - Dois Vizinhos – Paraná – Brazil
2Microbiology Laboratory, Department of Biological Sciences and Health, Paranaense University – UNIPAR, Unit of Francisco Beltrão, Av. Julio Assis Cavalheiro, 2000, Bairro Centro, Francisco Beltrão, Paraná, Brazil
Correspondence to: Sideney Becker Onofre, Department of Biological Sciences and Pharmacy, FAED - Faculty of Educational of Dois Vizinhos - União de Ensino do Sudoeste do Paraná – UNISEP - Av. Presidente Kennedy, 2601 –Bairro Nossa Senhora Aparecida - Dois Vizinhos – Paraná – Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Cellulases are enzymes of great industrial interest, which are used in the food, pharmaceuticals, cosmetics, detergents and textile industries. Applications include the bleaching of pulp in the paper industry, the production of dissolved pulp, waste water treatment and recycling of waste paper. Studies have been carried out regarding the ability of microorganisms to produce enzymes, using available and affordable substrates. The aim of this study was to evaluate the ability of the endophytic fungus Aspergillus flavus, strain (D2-FB) to produce cellulase. The studies were carried out using a substrate of sugarcane bagasse supplemented with 1% cellobiose and carboxymethylcellulose. The material was kept in an oven at 27º C for 69 days and the enzymes were measured every 7 days. To quantify the enzymes, the DNS method was adopted. The results showed that the highest production occurred at 32 days, with a production of 33.15 ± 7.96 U/g of substrate. After this period, the enzyme production decreased gradually up to 3.06 ± 0.53 U/g. Based on these results, it can be concluded that the endophytic fungus A. flavus, strain (D2-FB), is a producer of cellulases.
Keywords: Enzymes, Bioprocesses, Biotechnology, Fermentation
Cite this paper: Sideney Becker Onofre, Géssica C. Silva, Shaiana P. Mattiello, Deizi Groth, Ivair Malagi, Production of Cellulolytic Enzymes by Aspergillus flavus Using Solid State Fermentation Based on Sugarcane Bagasse, American Journal of Biochemistry, Vol. 3 No. 2, 2013, pp. 25-28. doi: 10.5923/j.ajb.20130302.01.
Article Outline
1. Introduction
- Enzymes are biological catalysts, consisting of protein molecules produced by living cells. These biocatalysts have a high catalytic activity and specific selectivity for the substrate[1].Cellulases are enzymes that are capable of breaking cellulose’s glycosidic bonds, resulting in the release of oligosaccharides, cellobiose and glucose[2]. These hydrolytic enzymes are used in the food, pharmaceuticals, cosmetics, detergents and textile industries. Their main applications include the bleaching of pulp in the paper industry, the production of dissolved pulp, waste water treatment and recycling of paper residues. Enzyme technology is currently one of the most promising new technology fields for the synthesis of valuable compounds.Industrial processes that employ enzymes in biotransformation processes have a lower environmental impact and concomitantly lower consumption of energy, since they are biodegradable and highly specific, which reduces undesirable effects[3,4].A wide variety of cellulase-producing microorganisms are present in nature, and they are capable of degrading natural cellulose. Cotton and filter paper, among others, are used as inducing substrates for the production of exo-glycosidases and for measuring the activity of the total cellulolytic complex[5]. Among the microorganisms, fungi are noteworthy for producing enzymes of industrial interest. Fungi are uni or multicellular eukaryotic organisms, which are heterotrophic, chemoorganotrophic, aerobic or microaerophilic; some have cell walls composed of chitin and cellulose[6]. The genus Aspergillus sp. is the most common filamentous fungus, and is one of the most thoroughly studied. The species of this genus are distributed worldwide and are present on the surface, in air and water, both in plant organisms and in animals, and are associated with the deterioration of plant materials and food. Many species of Aspergillus are used to obtain enzymes, in chemical biosynthesis and transformation of compounds[7].Agroindustrial wastes and low-cost cellulosic biomass can be used to produce cellulases, which not only greatly reduces the cost of production of these enzymes but also may result in a yield similar to that obtained with other carbon sources as well as contributing to the environment. Several agro-industrial residues can be used as a substrate, such as orange bagasse, wheat and rice bran, soybean, apple pulp, coffee pulp, sugar cane bagasse, passion fruit peel, pineapple bagasse and cashew apple pulp[2]. Bagasse from sugarcane has been used as a substrate for growing large numbers of microorganisms, including bacteria, yeasts and filamentous fungi. However, fungi are the most commonly used, due to the amount of enzymes and proteic enrichment that they produce. The bagasse has been used as a substrate for the production of cellulases and xylanases by various Aspergillus species, including A. niger and A. phoenicis[8,9].The objective of this study was to evaluate the ability of the endophytic fungus Aspergillus flavus, strain (D2-FB), isolated from Baccharis dracunculifolia D.C (Asteraceae) to produce cellulases.
2. Materials and Methods
2.1. Studied Microorganism
- This study used the endophytic fungus Aspergillus flavus, strain (D2-FB), isolated from Baccharis dracunculifolia D.C. (Asteraceae), isolated in the period 2008 to 2009 and kept in the fungi collection of the Microbiology Laboratory of the Universidade Paranaense - Unipar - Unidade Universitária de Francisco Beltrão - PR.
2.2. Determination of the Cellulolytic Activity
- For the determination of cellulolytic activity, the study used a basic support of sugarcane bagasse that was successively rinsed in water for complete removal of sugars. The rinsed pulp was dried in an oven with air circulation at 65 °C for 24 hours, and was then packed in a polyethylene bag and stored in a dry environment.
2.3. Fermentation in Erlenmeyer Flasks
- 1% cellobiose and 1% carboxymethylcellulose were added to the sugarcane bagasse substrate to induce the production of cellulases and as the medium’s initial carbon sources. This mixture was inoculated with a suspension of 5g of the fungus, previously grown on a rice medium. It was then homogenized in an Erlenmeyer flask and incubated at 28°C for 69 days.
2.4. Analysis of the Fermented Substrate
- Aliquots of five grams of the medium were collected every 7 days and mixed with 50 ml of distilled water in the presence of 7.0 buffer. This suspension was stirred continuously for 30 minutes. It was then filtered to remove solids to yield a clear extract used for pH measurement. The extract was centrifuged at 3000 rpm for 15 minutes and the supernatant was considered an enzyme source to determine reducing sugar via the indirect spectrophotometric method. The indirect spectrophotometric method was used to determine enzyme activity based on the release of glucose molecules by the action of the cellulolytic enzymes complex.
2.4. pH
- pH was measured on a suspension obtained after homogenization of 5g of ferment in 50 ml of distilled water, which was continuously stirred for 30 minutes.
2.5. Dosage of Reducing Sugars
- Reducing sugars were determined by the reaction with 3,5-dinitrosalicylic "DNS"[10]. In an alkaline medium and at elevated temperature, the 3,5-dinitrosalicylic turns into 3-amino-5-nitrosalicylic. It develops a yellowish coffee color that absorbs at 540 nm. One unit of cellulases was defined as the quantity of released enzyme capable of acting on the substrate and releasing one μmol of reducing sugar (expressed as glucose) per minute under the test conditions [11].
3. Results and Discussion
- The data obtained from the fermentation process using a substrate of sugarcane bagasse supplemented with 1% carboxymethylcellulose and cellobiose, inoculated with the endophytic fungus Aspergillus flavus, isolated from Baccharis dracunculifolia D. C. (Asteraceae), are shown Fig. 1.
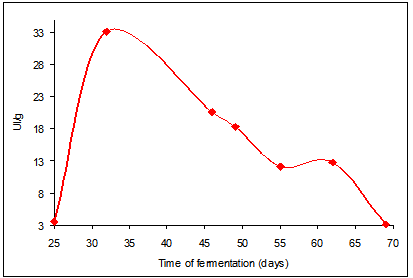 | Figure 1. Behavior of the endophytic fungus Aspergillus flavus, strain (D2-FB), in the production of cellulolytic complex in solid fermentation at a temperature of 28°C and pH of 6.54 |
4. Conclusions
- It can be concluded that the endophytic fungus Aspergillus flavus, strain (D2-FB) is able to produce enzymes of the cellulolytic complex. Therefore, its use in processes to obtain enzymes and produce energy sources such as glucose and cellobiose is extremely important. Sugarcane bagasse, used as substrate, was able to induce the expression of genes responsible for production of cellulases by the endophytic fungus Aspergillus flavus, strain (D2-FB), given that significant amounts of enzymes were obtained. The fermentation time that led to the highest production of the enzyme was 32 days, with a yield of 33.15±7.96 enzyme units/gram of substrate, at pH 6.82 and a temperature of 28°C.
ACKNOWLEDGEMENTS
- We would like to thank IBAMA - Brazilian Institute for the Environment, for the license for collection of biological material and the União de Ensino do Sudoeste do Paraná, for financing this project.
Conflict of Interest
- The authors declare no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML