-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(1): 18-23
doi:10.5923/j.ajb.20130301.03
Protective Effect of Bi-Herbal Formulation of Ocimum gratissimum and Gongronema latifolium Aqueous Leaf Extracts on Acetaminophen-induced Hepato-Nephrotoxicity in Rats
Ezeonwu V. U. , D. Dahiru
Department of Biochemistry, Modibbo Adama University of Technology, Yola, Adamawa State, Nigeria
Correspondence to: D. Dahiru , Department of Biochemistry, Modibbo Adama University of Technology, Yola, Adamawa State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Medicinal plants are used either as mono, bi or multi-herbal. The present study evaluated the protective effect of a bi-herbal formulation of Ocimum gratissimum and Gongronema latifolium aqueous leaf extracts in acetaminophen-induced liver and kidney toxicity in rats. Oral administrations of 200, 300 and 500 mg/kg body weight (bw) of the bi-herbal extract, 500 mg/kg bw each of mono extract and 100 mg/kg bw silymarin (reference drug) were administered to laboratory animals for 10 days. A suspension of acetaminophen at 750 mg/kg bw was administered once every 72 hours to induce toxicity. Biochemical analysis showed significant (p < 0.05) increase in the levels of Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (TB), creatinine (CRT), urea, cholesterol (CHOL) and triglyceride (TG) with a reduction in total protein (TP) levels of rats administered acetaminophen only. There was a dose dependent reversal of these changes in rats pretreated with 200, 300 and 500 mg/kg bw of the bi-herbal extract. The bi-herbal extract showed higher protective activity than the mono extracts of Ocimum gratissimum and Gongronema latifolium. The bi-herbal extract’s activity at 500mg/kg bw was comparable with 100 mg/kg bw silymarin. The study demonstrated that the aqueous bi-herbal extract of Ocimum gratissium and Gonglonema latifolium possessed better protective activity as against the mono extracts in acetaminophen-induced toxicity. This possibly suggested the combined use of the plants in traditional medicine.
Keywords: Bi-Herbal Extract, Ocimum gratissimum, Gongronema latifolium, Hepatoprotective, Nephroprotective, Silymarin, Acetaminophen
Cite this paper: Ezeonwu V. U. , D. Dahiru , Protective Effect of Bi-Herbal Formulation of Ocimum gratissimum and Gongronema latifolium Aqueous Leaf Extracts on Acetaminophen-induced Hepato-Nephrotoxicity in Rats, American Journal of Biochemistry, Vol. 3 No. 1, 2013, pp. 18-23. doi: 10.5923/j.ajb.20130301.03.
Article Outline
1. Introduction
- An excessive exposure to non-steroidal anti-inflammatory drug such as acetaminophen can induce liver and kidney damage and invariably affect the physiology of both organs in animals. Acetaminophen, an effective antipyretic and analgesic agent is one of the most used and abused drugs in the world. The toxicity of this substance stems from the intermediate produced during its metabolism by the cytochrome P450 enzyme system known as N-acetyl-p-benzoquinoneimine (NAPQI)[5, 26]. The liver and the kidney are among the most important internal organs in the body. Maintenance of life largely depends on how efficient both organs are since they are directly related to several metabolic processes. In view of the importance of these organs to the body, special attention must be devoted towards ensuring that substances capable of compromising the integrity and functions of these organs do not get into the body or are made less toxic. Ocimum gratissimum (O. Gratissimum) of the family Lamiaceae, commonly known as ‘scent leaf’ is a perennial plant, commonly used as spice in food. Scent leaf has been reported to be rich in plant chemicals. The plant is known to contain alkaloids, tannins, phytates, flavonoids and oligosaccharides[14]. The presence of phenols has been reported in the ethanolic extract of the plant[10]. Some of the reported pharmacological activities of the plant include antiseptic, antibacterial, and antifungal[11]. O. gratissimum has proved to be an effective anti-microbial agent at very low concentrations[21]. The plant is used in Nigeria by traditional medicine practitioners for the treatment of various diseases including epilepsy, diarrhoea, mental illness and fever[32]. There have been speculations that scent leaves may possess hypoglycemic activity in streptozotocin-induced diabetic[9]. Njoku et al. gave the LD50 of the herb as 2450 mg/kg body weight[19] Gongronema latifolium (G. Latifolium) of the family Asclepiadaceae, locally known as ‘utazi’ in the Igbo dialect of the eastern Nigeria is a tropical rainforest plant primarily used as spice and vegetable in the traditional folk practice[34, 35]. Phytochemical evaluation of the plant has shown that it is rich in essential oils, saponins and pregnanes amongst others[17, 28]. Alkaloids, tannins, phytates, flavonoids and oligosaccharides were identified in phytochemical analysis of the plant[18]. Traditionally, the southern part of Nigeria uses this herb in the treatment of malaria, diabetes and hypertension as well as a laxative. Nwanjo et al reported the anti lipid peroxidative activity of the plant[20]. Ugochukwu et al reported that the aqueous and ethanolic extracts of G. latifolium had hypoglycemic, hypolipidemic and antioxidative properties[34]. In the face of such report, the herb can be seen as a life saver considering the prevalence of such disease conditions. It was reported that the plant has anti-inflammatory and antimicrobial properties[18, 34]. Effiong et al reported the LD50 of the herb as 5000 mg/kg by oral route and 1500 mg/kg intraperitoneally[8]. Medicinal plants are important sources of drugs for the treatment of several ailments. The plants can be used alone or combined with other plants[3]. Several plants have been associated with nutritional and therapeutic benefits. Ocimum gratissimum and Gongronema latifolium are among such plants proven to have both nutritional and medicinal properties. Both plants traditionally are normal constituents of food taken predominantly in the eastern part of Nigeria and on several occasions used for the treatment of diseases. Combination of herbs to achieve a desired aim has been practiced along different cultures and in trado-medical practice. This crude form of combinatorial chemistry is not new and the practitioners have several motives to combine herbs. Amongst the reasons for such combination is the possibility of a synergic activity of both herbs, the moderation of effects or activity of one herb using another by reducing the toxicity and undesired effects. Therefore, studies involving the combination of two or more different herbs are promising area of research. This study investigated the effects of a bi-herbal aqueous extract of Ocimum gratissimum and Gongronema latifolium in acetaminophen-induced toxicity.
2. Materials and Methods
2.1. Animals
- Forty albino rats (100-110g) obtained from the animal house of an independent farmer within Adamawa State, Nigeria were used for this study. The animals were housed in wooden cages. They were allowed to acclimatize to the new environment for 3days before the commencement of treatment. Throughout the period of the experiment the animals were fed normal feed from Pfizer feeds. Acetaminophen was administered to rats 30 min after extract administration. The experiment was carried based on the approved institutional guidelines for the care and handling of laboratory animals.
2.2. Plant Material
- Fresh O. gratissimum and G. latifolium leaves were collected from a donor garden and authenticated at the department of Plant Sciences of Modibbo Adama University of Technology, Yola.
2.3. Preparation of Extract
- Both plant materials were shade dried for 12days and made into powder and then sieved. Equal amount of both plants (250 g each plant) was soaked in 1000 ml of distilled water in a beaker, the mixture was shaken and allowed to stand for 24 hours, boiled for 5minutes before filtering with a cheese cloth. The filtrate was evaporated using an oven at 50-60°C. Appropriate weights of the residue were prepared in distilled water to obtain the various concentrations used for the experiment. Each herb was also prepared separately following similar pattern described except the fact there was no combination of both herbs.
2.4. Experimental Design
- Eight groups of five animals were used in the study. The study design by Hiroshini et al[13] was adopted. Each group was treated as follows:

2.5. Blood Collection
- Blood was collected 12 hours after the last dose was administered. The animals were sacrificed and whole blood was collected by cardiac puncture into heparinised bottles. While another portion of the blood was collected in ordinary bottles allowed standing for 30 min to clot and thereafter centrifuged at 2500 rpm for 10min. Serum samples and heparinised plasma were used in the study depending on a given test.
2.6. Biochemical Analysis
- The colorimetric end-point method described by Reitman and Frankel[25] was used in assaying for aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) levels in the samples while the p-nitrophenol method was used to evaluate alkaline phosphatase (ALP)[6]. Also, the Jendrassik and Grof method described by Doumas et al[7] was used in assaying for direct bilirubin levels. Total protein levels were determined using Biuret method described in Reinhold (1953). The Diacetyl monoxime method described in Wybenga et al[37] was used to evaluate urea level. The Jaffe’s method[31]was used to evaluate the creatinine (CRT) levels. The Randox standard kits (Randox Limited, UK) were used to assay for the above named parameters including fasting blood sugar (FBS), cholesterol (CHOL), total bilirubin (TB) and triglyceride (TG).
2.7. Statistical Analysis
- All values are expressed as Mean ± SEM (Standard Error of Mean). The data was analyzed using Student T-test at P < 0.05 level of significance to confirm significant difference between two means.
3. Results
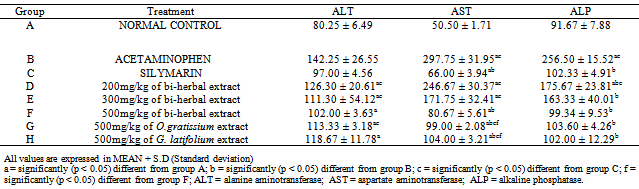
- Administration of acetaminophen to rats resulted in significant (p < 0.05) increase in the levels of enzyme markers (ALT, AST and ALP) of tissue damage (142.25 ± 26.55, 297.75 ± 31.95 and 256.50 ± 15.52 respectively) when compared to values of normal control rats (80.25 ± 6.49, 50.5±1.71 and 91.67 ± 7.88 respectively). Pretreatment of rats with bi-herbal extracts at doses of 200, 300 and 500 mg/kg bw were found to significantly (p < 0.05) decrease the levels of these enzymes when compared to values of rats administered acetaminophen only. Pretreatment with individual extracts at a dose of 500 mg/kg bw also inhibited the raised levels of these enzymes when compared to acetaminophen group (Table 1).
|
|
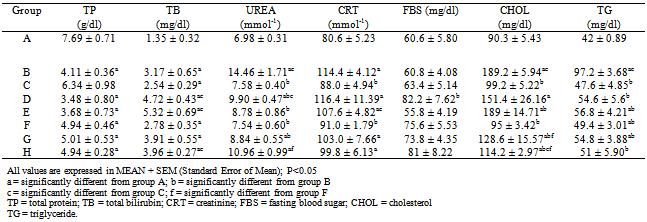
- Table 2, shows the effect of pretreatment of rats with bi-herbal and individual extracts on non enzyme markers of tissue damage in acetaminophen-induced tissue toxicity. Administration of acetaminophen significantly (p < 0.05) increased the levels of TB (3.17 ± 0.65), urea (14.46 ± 1.71), CRT (114.4 ± 4.12), CHOL (189.2 ± 5.94) and TG (97.2 ± 3.68) with significant decrease in the level of total protein (4.11 ± 0.36) when compared to values of normal control rats (1.35 ± 0.32, 6.98 ± 0.31, 80.6 ± 90.3 ± 5.43, 42 ± 0.89 and 7.69 ± 0.71 respectively). Pretreatment with bi-herbal, silymarin and individual extracts remarkably reversed to various degrees the effects produced by acetaminophen administration.
4. Discussion
- Liver enzymes, ALT, AST, and ALP are usually low in normal control. Injury to the liver results in the release of these enzymes into the blood. An elevated level of these enzymes markers of hepatic necrosis in animals treated with only acetaminophen indicates tissue damage. Injury to the hepatocytes changes their transport function and membrane permeability, causing leakage of enzymes from the cells[1]. Consequently, the significant release of these enzymes and their increased activity in animals treated with acetaminophen can be interpreted to be as a result of liver cell destruction and alteration in the membrane permeability[12]. Increased bilirubin content reflects the pathophysiology of the liver and one of the most sensitive and useful test to substantiate the functional integrity of the liver and severity of necrosis which measures the binding, conjugating and excretory capacity of hepatocytes that is proportional to the erythrocytes degradation rate[30]. Increased levels of bilirubin reflect the depth of jaundice and increased aminotransferases and alkaline phosphatase was the clear manifestation of cellular leakage and loss of functional integrity of the cell[27]. The bi-herbal extract showed a dose dependent activity in reducing the levels of these enzymes. Silymarin, a standard drug used in the treatment of liver injury at 100 mg/kg showed no significant difference in activity when compared to group treated with 500 mg/kg bi-herbal extract. The reversal of increased serum enzymes in acetaminophen-induced liver damage by the extract may be due to its membrane stabilizing activity thus preventing the leakage of intracellular enzymes. The protective activity of individual extracts of O. gratissium and G. latifolium confirms previous reports[9, 34]. The effective control of total protein and total bilirubin can be attributed to an improvement in the hepatic cells’ secretory mechanisms. The efficacy of any hepato-protective drug is dependent on its capacity of either reducing the harmful effect or restoring the normal hepatic physiology that has been disturbed by a hepatotoxin[23]. Both silymarin and the bi-herbal extract decreased acetaminophen induced elevated enzyme levels in tested groups, indicating the protection of structural integrity of hepatocytes, its cell membrane or regeneration of damaged liver cells. Several metabolic disorders including urea and creatinine derangements are possible in the presence of acetaminophen over dosage[15]. Increased concentration of serum urea and creatinine are considered for investigating drug induced nephrotoxicity in animals and man[4]. Acetaminophen treatment obviously interfered with kidney filtration functions as seen by its elevated values in rats. Urea, a waste product of protein catabolism can rise when the kidney is defective. In renal disease, the serum urea accumulates because the rate of serum urea production exceeds the rate of its clearance[16]. The elevation of urea and creatinine levels in the serum is taken as the index of nephrotoxicity[22, 2, 16]. The bi-herbal extracts had a dose dependent reversal of the effects on this parameter. The present study showed the nephroprotective effects of the bi-herbal extract of O. gratissimum and G. latifolium in acetaminophen-induced toxicity.There was an appreciable increase in the cholesterol levels of the acetaminophen treated group when compared with the normal group. The liver is central to the regulation of cholesterol levels in the body, not only does it synthesize cholesterol for export to other cells but it also removes cholesterol from the body by converting it to bile salts and excreting it into the bile. Levels of triglyceride in the serum or liver could increase due to several processes, including increased availability of free fatty acid, glycerophosphate, decreased VLDL in the serum and decreased removal of triglyceride and cholesterol from serum due to diminished lipoprotein activity[33, 38]. The extracts showed a dose dependent reversal of such increased. The bi-herbal extract, being able to show more efficacy than the individual makes a case for the use of bi-herbal extracts. Also, the triglyceride levels obtained from the study showed a significant decrease across the group. Phytochemical studies of O. gratissimum and G. latifolium showed that both herbs are rich in flavonoids[14, 18]. Flavonoids have been widely reported to be hepato-protective[29, 36]. The observed hepatoprotective activity of the bi-herbal extract may be due to the presence of flavonoids.
5. Conclusions
- The present study demonstrated that both O. gratissimum and G. latifolium extracts possessed hepatoprotective and nephroprotective properties. The combined use of both plants (bi-herbal) produced better activity at protecting the animals against acetaminophen-induced toxicity than the individual plants. Administration of the bi-herbal extracts at 500 mg/kg bw was found to compare favourably with the reference drug silymarin at protecting the animals against acetaminophen-induced toxicity. There is need to explore the possible mechanisms by which the bi-herbal extract protects the animals.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML