-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(1): 6-17
doi:10.5923/j.ajb.20130301.02
Amelioration of Hepatic Lipid Peroxidation and Insulin Sensitivity by an Ethanolic Extract of Moringa oleifera in Non-Alcoholic Fatty Liver Disease (NAFLD) in Rats
Ebuehi Osaretin A. T., Alli-balogun Ganiyu O
Department of Biochemistry, College of Medicine, University of Lagos, P.M.B. 12003, Lagos, Nigeria.
Correspondence to: Ebuehi Osaretin A. T., Department of Biochemistry, College of Medicine, University of Lagos, P.M.B. 12003, Lagos, Nigeria..
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
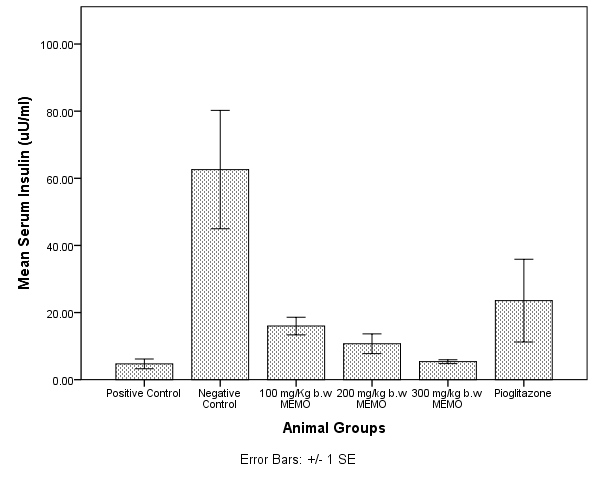
The study is to evaluate the potential of ethanolic extract of Moringa oleifera (EEMO) in ameliorating biochemical anomalies associated with Non-Alcoholic Fatty Liver Disease (NAFLD). Rats were fed 5 ml of high fat emulsion (HFE) for three weeks to induce NAFLD. Rats in Groups 3,4,5,and 6 were administered 5ml of high fat emulsion (HFE) daily for 3 weeks and subsequently administered 100, 200, 300 mg/kg of EEMO and 30 mg/kg pioglitazone (PG) respectively. NAFLD induction resulted in significant increase in hepatic and serum triacylglycerols (TAG) and cholesterol (CHOL) levels when compared with control, while treatment with EEMO resulted in significant reduction. NAFLD induction had no significant effects on hepatic reduced glutathione (GSH) and catalase activity. There was a significant increase in activity of Superoxide Dismutase (SOD) and malondialdehyde (MDA) level, signifying the existence of hepatic lipid peroxidation. There was also a significant increase in Homeostasis Model Assessment for insulin resistance (HOMA-IR), serum insulin and fasting blood glucose (FBG) levels, suggesting diminished insulin sensitivity. Administration of EEMO resulted in decrease SOD activity in a dose dependent fashion when compared with FBG. A similar effect was seen with the levels of MDA, insulin, FBG and HOMA-IR. These findings suggest that EEMO improves the hepatic status of animals with NAFLD.
Keywords: HFE, NAFLD, Moringa oleifera, Insulin Sensitivity, Lipid Peroxidation
Cite this paper: Ebuehi Osaretin A. T., Alli-balogun Ganiyu O, Amelioration of Hepatic Lipid Peroxidation and Insulin Sensitivity by an Ethanolic Extract of Moringa oleifera in Non-Alcoholic Fatty Liver Disease (NAFLD) in Rats, American Journal of Biochemistry, Vol. 3 No. 1, 2013, pp. 6-17. doi: 10.5923/j.ajb.20130301.02.
Article Outline
1. Introduction
- The term fatty liver (also called steatosis or hepatosteatosis) refers to a condition characterized histologically by accumulation of triacylglycerol within the cytoplasm of hepatocytes[1]. The presence of hepatosteatosis in the absence of excessive alcohol consumption is termed a non-alcoholic fatty liver disease (NAFLD). NAFLD comprises a spectrum of disorders which range from simple steatosis to inflammatory steatohepatitis [2]. NAFLD is considered to be a manifestation of the “metabolic syndrome”, a constellation of various anomalies involving insulin resistance (IR), visceral obesity, dyslipidaemia, diabetes, hypertension and other factors[3]. In actual fact, NAFLD is gradually becoming the most common cause of liver disease in western countries and is now recognised to be the aetiology in many cases previously tagged as cryptogenic cirrhosis[4]. A plausible explanation for the pathogenesis of NAFLD is based on the ‘two hit’ hypothesis which was proposed in 1998[5]. The first step which is also referred to as the first hit is characterized by hepatic fat accumulation or steatosis which increases vulnerability of the liver to injury mediated by ‘second hits’ which include inflammatory cytokines/adipokines, mitochondria dysfunction and oxidative stress which in turn lead to steatohepatitis and/or fibrosis[5,6]. Several therapeutic agents are used in the treatment of NAFLD and associated disorders, but recently more attention has been shifted to the use of plants as a possible means of alleviating the symptoms associated with NAFLD[7]. Moringa oleifera (MO), belonging to the family Moringaceae, commonly known as the drumstick or horseradish tree in English is a widely known plant in different parts of south Asia and Africa. In traditional medicine, the leaves are used for the treatment of disorders. The research carried out by Ghasi et al.[8] revealed that the leaves possess lipid lowering activity in high fat diet fed rats. In addition, a research carried out on the aqueous extracts showed that the leaves possess hypoglycemic properties. In that study, the aqueous leaf extracts were shown to lower the blood sugar in diabetic animals[9, 10]. Das et al.[7] reported that an ethanolic extract of MO possessed hepatoprotective action coupled with restoration of antioxidant status in rats fed with a high fat diet. Also, Sinha et al.[11] demonstrated that a leaf extract ameliorated ionizing radiation induced lipid peroxidation in rats. In this present study, a classic experimental model of NAFLD and IR in Wister rats was utilized. This model is characterized by clinical pathophysiological characteristics of NAFLD and IR based on utilization of glucose by the body, steatosis and alterations of various cellular and molecular events related to NAFLD and IR.
2. Materials and Methods
2.1. Plant Sample Preparation
- Fresh leaves of Moringa oleifera were obtained from the Faculty of Agriculture and Forestry, University of Ibadan, Ibadan, Nigeria. A voucher specimen was authenticated and deposited at the Herbarium, of the Department of Botany, University of Lagos, Akoka, Lagos with herbarium number LuH 5061. The leaves were shade dried for five days and then ground into a coarse powder. About 500g of the powder was macerated with 2 litres of 99.7% ethanol at room temperature for 48 hours with occasional shaking. The resulting extract was filtered using a filter paper (Whatmann No.1) and the resulting filtrate was concentrated using a rotary evaporator under reduced pressure to give a semi-solid residue which was then lyophilized to get a brownish powder weighing 34.50g (yield 6.90%, w/w) was obtained.
2.2. Preparation of Fat Emulsion
- This was done according to the method described by Ai et al.[12] with a few modifications. Briefly, a constant volume of 100 ml emulsion shall contain 20g lard (source of saturated fat), 5g cholesterol, 1g sodium glutamate, 5g sucrose, 20 ml Tween 80 and 30 ml propylene glycol shall be prepared by adding distilled water and storing at 4℃.
2.3. Experimental Animals
- Forty eight[48] adult male rats of the Sprague-Dawley strain weighing about 150-180g were used for the NAFLD experiment while 50 Swiss albino mice were used for the LD50 experiment. The animals were acclimatized for seven days at a temperature of 25℃. The animal room was regulated at a 12 hour light; 12 hour dark schedule. All animal experiments were carried out according to the institution’s ethics.
2.4. Experimental Design
- Six different groups containing eight animals each were used for the study. Animals in group 1 served as the positive control group. These animals had free access to food and water. Animals in group 2 served as the Negative control. These animals were administered 5ml of the high fat emulsion for 35 days. Animals in groups 3, 4, 5 and 6 were given a similar treatment but after the twenty first day, they were administered varying doses of 100, 200, 300 mg/kg body weight of EEMO and 30 mg/kg body weight Pioglitazone respectively. On the twenty first day, two animals were drawn randomly from each group and sacrificed; livers were excised and subjected to histopathological examination for the confirmation of hepatosteatosis. On the penultimate day of the experiment, the animals were fasted overnight and their fasting blood glucose was measured on the 35th day. Blood was collected into universal sample bottles by ocular puncture and the animals were subsequently sacrificed by cervical decapitation with excision of liver afterwards.
2.5. Preliminary Phytochemical Screening
- The ethanolic extract of MO (EEMO) was subjected to various tests to determine the presence of phytochemicals which include; alkaloids, phenolic compounds, flavonoids, terpenes, saponins and steroids using standard methodology [13].
2.6. Quantitative Phytochemical Analysis
2.6.1. Determination of Tannin Content
- Tannin content was determined by Vanillin hydrochloride method[14]. Vanillin hydrochloride reagent was prepared by mixing equal volumes of 8% HCl in methanol and 4% vanillin in methanol. A volume of 1.0 ml of 0.1 mg/ml of extract solution was mixed with 5 ml vanillin hydrochloride reagent; the mixture was allowed to stand for 20 min. The absorbance was measured at 500 nm. Extract samples were evaluated at a final concentration of 1 mg/ml. Total tannin content was expressed as rutin equivalents (mg/g) using the following equation based on calibration curve: y = ax + b, where x was the absorbance and y was the rutin equivalent (mg/g).
2.6.2. Determination of Total Phenolic Content
- The content of the total phenolic in plant extract is determined by Folin Ciocalteu method[15] spectrometrically. To 1 ml of Folin-Ciocalteu’s reagent, previously diluted (1:20) was added to 1 ml of sample (250 μg/ml) and mixed thoroughly. To the mixture, 4 ml of sodium carbonate (75 g/L) and 10 ml of distilled water were added and mixed well. The mixture was allowed to stand for 2 h at room temperature. Contents were then centrifuged at 2000 g for 5 min and the absorbance of the supernatant was taken at 760 nm. A standard curve was obtained using various concentrations of gallic acid. Results were expressed as percentage of gallic acid equivalents (GAE).
2.6.3. Determination of Flavonoid Content
- This was done according to the method of Bohm and Kopa-Bazan[16]. 1 g of plant extract was mixed with 10 ml of 80% aqueous methanol at room temperature. The whole solution was filtered through a Whatman filter paper # 42 (125 mm). The filtrate was later transferred into a crucible and evaporated into dryness over a water bath, the weight of the material and percentage quantity was calculated.
2.6.4. Assay for DPPH Free Radical Scavenging Ability
- Radical scavenging activity of the ethanolic extract of M. oleifera was measured by a modified DPPH method[17]. DPPH(2,2-diphenyl-1-picrylhydrazyl-1,1-diphenyl-2-picrylhydrazyl) in ethanol is a stable radical, dark violet in color. Its color is bleached by its reaction with a hydrogen donor (derived from plant sample under scrutiny).
2.7. Acute Toxicity Study
- This was performed according to OECD-423 guidelines[18]. Albino mice were fasted overnight and were afterwards, administered with the ethanolic extract by gavage at a single dose of 15mg/kg body weight. The animals were observed for seven days. No mortality was however recorded. The procedure was also done for higher doses such as 50, 100, 200, 400, 800, 1600 mg/kg body weight.
2.8. Assessment of Hepatic and Serum Lipid Profiles
- Total cholesterol (TC) and Triacylglycerols (TAG) were determined colorimetrically in both liver homogenates (supernatant) and sera using commercially available kits (Randox laboratories, N Ireland). For High-density Lipoprotein cholesterol determination, low-density lipoproteins (LDL and VLDL) and chylomicron fractions in the sample were precipitated quantitatively by the addition of phosphotungstic acid in the presence of magnesium ions. The mixture was allowed to stand for 10 minutes at room temperature centrifuged for 10 minutes at 4000rpm. The supernatant represented the HDL-Cholesterol fraction. The cholesterol concentration in the HDL fraction, which remained in the supernatant, was determined. The serum LDL and VLDL-cholesterol levels were estimated using the Friedwald’s formula[19], where LDL-Cholesterol is given by the relations:
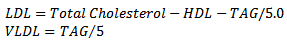
2.9. Determination of Insulin Sensitivity
- Serum insulin levels were estimated using a commercially available Rat Insulin ELISA kit (Crystal Chem INC. USA). Insulin sensitivity was determined using the Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) index using the formula below. Higher values of HOMA-IR signify elevated peripheral insulin resistance[20].HOMA-IR= Fasting plasma insulin (μU/ml) x Fasting glucose (mmol/L) / 22.5
2.10. Assessment of Hepatic Lipid Peroxidation and Antioxidant Status
- Superoxide dismutase (SOD) activity was assayed according to the method of Kakkar[21]. A single unit of enzyme was expressed as 50% inhibition of NBT (nitroblue tetrazolium) reduction/min/mg protein. Catalase (CAT) activity was assayed colorimetrically at 620nm and expressed as µmoles of hydrogen peroxide consumed/min/ mg protein as described by Sinha[22]. Hepatic levels of reduced glutathione (GSH) was determined according to the method of Ellman[23] while hepatic malondialdehyde (MDA) levels were estimated using the thiobarbituric acid (TBA) method.
2.11. Histopathological Examination of Tissues
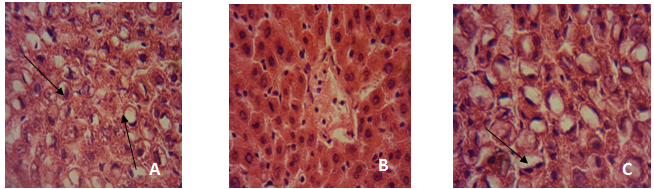
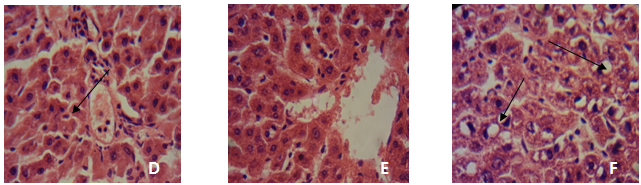
- After rats were sacrificed, the liver of a rat from each group was fixed in 10% formol saline and after 72 h the organs were dehydrated in graded alcohol, cleared in xylene, and embedded in paraffin. The resulting blocks were exhaustively sectioned. The sections were randomized and selected sections were stained in haematoxylin and eosin. The slides were then examined at magnification of × 100 under a light microscope. Photomicrographs were taken using a Sony cybershot digital camera.
2.12. Statistical Analysis
- The results are expressed as Mean ± S.D. and all statistical comparisons were made by means of one-way analysis of variance (ANOVA) followed by Tukey’s Lowest significance difference (LSD). Data were analysed with SPSS 20.0 (IBM) and Graph Pad Prism 5.0v for Windows (Graph Pad Software, San Diego, CA, USA). The difference showing a Probability (p) level of 0.05 or lower was considered to be statistically significant.
3. Results
3.1. Preliminary Phytochemical Screening
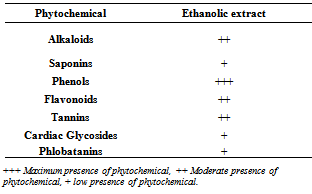
- Phytochemical screening of EEMO leaves revealed the presence of various biologically active compounds of which alkaloids, phenolics, flavonoids, tannins and cardiac glycosides are the most prominent (Table 1).
|
3.2. Total Phenol, Flavonoid and Tannin Content
- Table 2 shows the total phenolic, flavonoid and tannin content of the ethanolic extract of M. oleifera used in this study. The total phenolic content was estimated as 0.727±0.414 mg/g, flavonoid content as 0.858±0.213 mg/g while total tannins were estimated as 0.244±0.009 mg/g.
|
3.3. DPPH Radical Scavenging Activity (% Inhibition)
- Figure 1 shows the DPPH free radical scavenging activity of MEMO in comparison with Ascorbic acid (AA) and butylated Hydroxytoluene (BHT). At a concentration of 25µg/ml, the scavenging activity of EEMO was 50.71% while ascorbic acid and BHT at the same concentration had 96.23 and 44.2% respectively. At a concentration of 50µg/ml EEMO, AA and BHT had scavenging activities of 52.08%, 96.23% and 50.33% respectively. At a concentration of 75µg/ml EEMO, AA and BHT had scavenging activities of 67.29%, 97.41% and 56.84% respectively. At a concentration of 100µg/ml EEMO, AA and BHT had scavenging activities of 89.83%, 97.46% and 58.83% respectively.
3.4. Acute Toxicity
- At 15 mg/kg body weight, mortality was not observed. Mortality was not noticed up to 400 mg/kg, whereas, the LD50 of the extract was found to be 1800 mg/kg body weight. Toxic symptoms for which the animals were observed for 72 h include behavioural changes, locomotion, convulsions and mortality.
3.5. Animal Weights
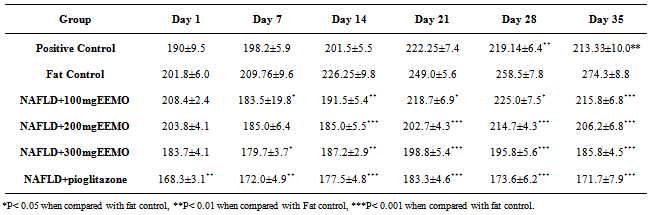
- Table 3 shows the weight distribution of the animals from the beginning of the experiment through the induction of NAFLD (Day 1-21), administration of EEMO (Day 22-34) and sacrifice (Day 35).
3.6. Fasting Blood Glucose
- Figure 2 depicts the fasting blood glucose (FBG) of animals on the last day of the experiment. When compared with the fat control group (124.7±2.08 mg/dl), there was a significant difference (p< 0.05) in the concentration of FBG in the positive control (73.83±6.91 mg/dl), 100mg/kg EEMO group (88.83±3.74 mg/dl), 200mg/kg EEMO group (79.83±2.79 mg/dl), 300mg/kg EEMO group (77.33±4.68 mg/dl), and the pioglitazone group (67.67±1.89 mg/dl). When compared with the positive control, the 100mg/kg EEMO group had a significantly higher FBG (p< 0.05) while the pioglitazone group recorded a significantly lower FBG when compared with the 100mg/kg and 200mg/kg EEMO groups (p< 0.05).
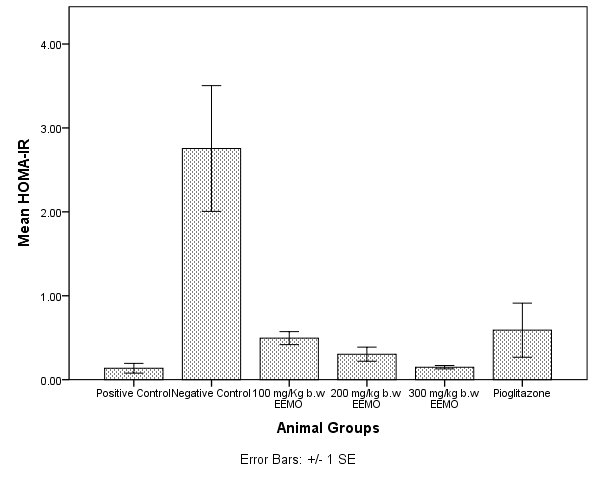
3.7. Homeostatic Model Assessment Index for Insulin Resistance (HOMA-IR)
- Figure 3 depicts the Homeostatic model assessment index for Insulin resistance (HOMA-IR) in the experimental animals. The Negative control group significantly had a higher value for HOMA-IR when compared with every other group (p< 0.05), with no significant difference amongst other groups (p> 0.05).
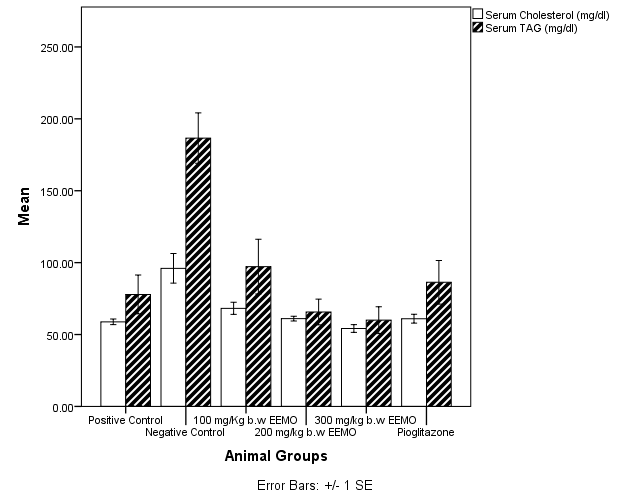
3.8. Serum and Hepatic Lipid Profiles
- Figure 4 depicts the serum Cholesterol (CHOL) and Triacylglycerol (TAG) levels in the experimental animals on the last day of the experiment. When compared with the negative control group (96.01±10.29 mg/dl), there was a significant difference (p< 0.05) in the concentration of CHOL in the positive control (58.8±1.95 mg/dl), 100mg/kg EEMO group (68.22±4.19 mg/dl), 200mg/kg EEMO group (61.07±1.64 mg/dl), 300mg/kg EEMO group (54.16±2.69 mg/dl), and the pioglitazone group (61.00±3.09 mg/dl). Similarly, When compared with the negative control group (186.62±17.53 mg/dl), there was a significant difference (p< 0.05) in the concentration of TAG in the positive control (78.9±13.5 mg/dl), 100mg/kg EEMO group (97.24±46.63 mg/dl), 200mg/kg EEMO group (65.65±8.97 mg/dl), 300mg/kg EEMO group (59.97±9.38 mg/dl), and the pioglitazone group (86.32±15.19 mg/dl).
|
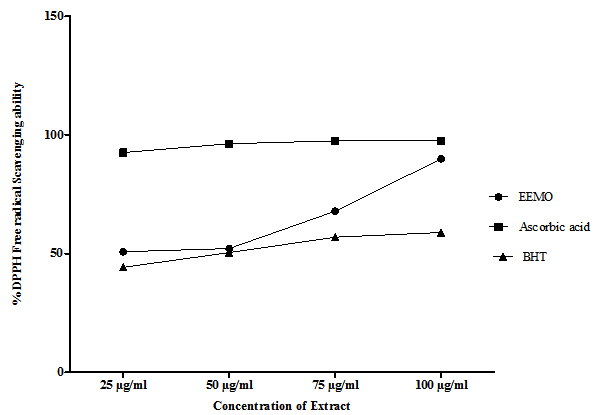 | Figure 1. DPPH Free Radical scavenging activity of the Ethanolic extract of M oleifera in comparison with Ascorbic acid and Butylated Hydoxy-Toluene (BHT) |
 | Figure 2. Fasting blood glucose levels (mg/dl) in various experimental groups |
 | Figure 3. Homeostatic Model Assessment values for Insulin Resistance (HOMA-IR) amongst all animal groups |
 | Figure 4. Serum Cholesterol and Triacylglycerol levels (mg/dl) in rats |
3.9. Serum Lipoprotein (HDL, LDL AND VLDL) Cholesterol
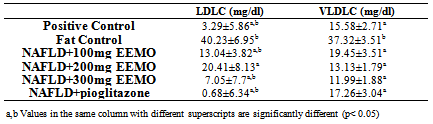
- Figure 4 shows the mean HDL-Cholesterol (HDLC) for the experimental animals. Compared with the Negative control, the concentrations of LDLC were significantly higher in the in the positive control (3.29± mg/dl), 300mg/kg EEMO group (19.86±3.38 mg/dl), and the pioglitazone group (21.1±3.12 mg/dl) (p< 0.05). There was no significant difference in HDLC concentration when compared with 100mg/kg EEMO group (12.96±1.95 mg/dl) and 200mg/kg EEMO group (13.41±0.68 mg/dl) (p> 0.05) while the positive control had a significantly higher HDLC when compared with the 100mg/kg EEMO group (p< 0.05).Table 3 shows the mean LDL-Cholesterol (LDLC) and VLDL-Cholesterol (VLDLC) for the experimental animals. Compared with the Negative control, the concentrations of LDLC were significantly lower in the in the positive control (3.29±5.86 mg/dl), 100mg/kg EEMO group (13.04±3.82 mg/dl) and 200mg/kg EEMO group (20.41±8.13 mg/dl) 300mg/kg EEMO group (7.05±7.7 mg/dl), and the pioglitazone group (0.68±6.34 mg/dl) (p< 0.05). Also, the pioglitazone group has a significantly lower LDLC when compared with 200mg/kg EEMO group, while the 200mg/Kg EEMO group had a significantly higher LDLC when compared with the positive control (p< 0.05). Compared with the Negative control (37.32±3.51 mg/dl), the concentrations of LDLC were significantly lower in the in the positive control (15.58±2.71 mg/dl), 100mg/kg EEMO group (19.45±3.51 mg/dl) and 200mg/kg EEMO group (13.13±1.79 mg/dl) 300mg/kg EEMO group (11.99±1.88 mg/dl), and the pioglitazone group (17.26±3.04 mg/dl) (p< 0.05).
3.10. Hepatic Total Cholesterol and Triacylglycerols
- Figure 5 depicts the concentration of hepatic Total Cholesterol (TC) and Triacylglycerols (TAG). The negative control had a significantly higher TC and TAG when compared with the other treatment groups.
3.11. Hepatic Lipid Peroxidation and Antioxidant Status
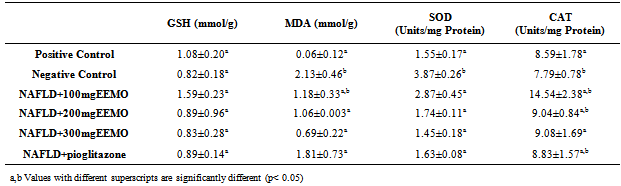
- Table 4 represents the hepatic levels of reduced GSH and Malondialdehyde MDA as well as the activities of antioxidant enzymes, SOD and catalase CAT. NAFLD induction had no significant effects on GSH and the activity of CAT (p> 0.05). There was however a significant increase in the activity of SOD and levels of MDA.
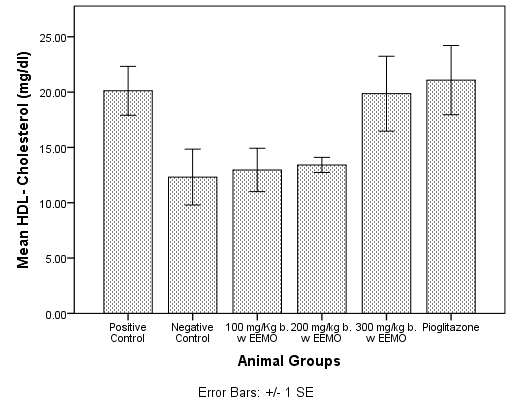 | Figure 5. HDL-Cholesterol Concentration in sera of rats |
|
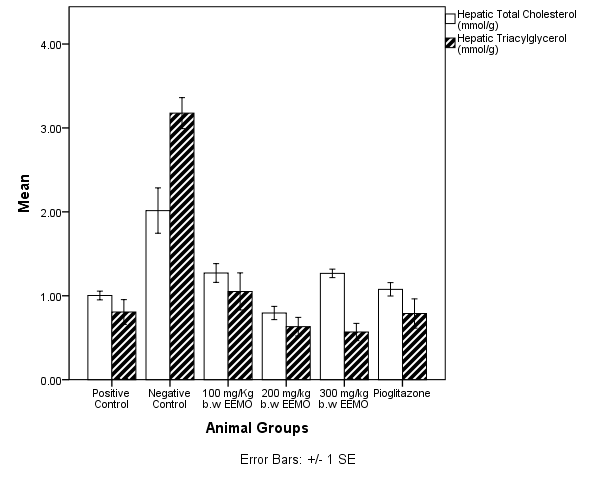 | Figure 6. Total Hepatic Cholesterol and Triacylglycerol in experimental animals |
|
3.12. Histopathological Findings
4. Discussion
- This study was designed to investigate the potentials of an ethanolic extract of M.oleifera in reversing several physiological conditions that occur as a result of the pathogenesis of a Non-Alcoholic fatty liver disease. NALFD induction via feeding with a high-fat emulsion resulted in a significant increase in the body weight of animals as seen in the Negative control group from day 7 of the experiment while administration with EEMO was found to result in a significant weight reduction as seen in the three dosage groups. This suggests that the extract reduces fat accumulation which ultimately results in hepatosteatosis (accumulation of fat in the liver). Data from this present study reveal that Fasting serum glucose was significantly increased as a result of NAFLD induction in the experimental animals. This was accompanied with an increase in serum insulin levels and an elevated HOMA-IR index when compared with the positive control. Diabetes mellitus is a pathological state which affects the whole system with the skeletal muscle, adipose and liver being the most affected[24, 25]. Alterations in the functional status of the liver and other mentioned tissues may result in the development of insulin resistance. A major manifestation of IR is the alteration of glucose absorption and utilization, metabolic disturbance of glucose in hepatocytes and lipoprotein metabolism disturbance in adipocytes[26]. Central to the pathogenesis of NAFLD is insulin resistance. It is almost universally demonstrated in patients with NAFLD[25, 28]. In one study, patients who had had simple steatosis and one who had Non-Alcoholic Steatohepatitis (NASH), insulin resistance was associated with NAFLD, independent of BMI or glucose tolerance[29].`Administration with the ethanolic extract of M. oleifera leaves was found to ameliorate hyperglycemia and insulin resistance in the experimental animals as there were no statistically significant differences in fasting serum glucose, insulin and HOMA-IR when treatment groups administered 200 and 300 mg/kg b.w EEMO were compared with the positive control group. Similarly, there were no significant differences when compared with the group administered with Pioglitazone. The results of this study are in agreement with the study of Divi et al.[26] in which an aqueous extract of M. oleifera was reported to normalize fasting blood glucose in fructose induced insulin resistance. A characteristic feature of NAFLD histologically and metabolically is the accumulation of TAG in the liver[25]. High fat feeding resulted in significantly greater hepatic fat accumulation in the NAFLD group when compared with other groups. This was revealed via the hepatic TAG and CHOL thereby suggesting the presence of hepatosteatosis. This was further supported by the histopathological examination in which the NAFLD group had visible fatty changes in the hepatocytes. Administration with EEMO was found to be effective in reversing these fatty changes with the 200 and 300 mg/kg b.w doses proving to be efficient. In comparison, pioglitazone resulted in a reversal of these fatty changes but was not complete as depicted in the micrographs. Serum TAG and CHOL were also significantly elevated as a result of high fat feeding in the Negative control while administration with EEMO was found to normalize the serum levels of these lipids. More so, there was a significant elevation in the levels of LDLC and VLDLC coupled with significantly lower HDL-Cholesterol levels. This suggests the contribution of dyslipidemia to the pathogenesis of NAFLD. Moringa oleifera leaves are utilised in native medicine for their lipid lowering effect. An aqueous extract of M. oleifera leaves prevented atherosclerotic plaque formation in artery and also possessed lipid lowering activity in rabbits, fed with a high cholesterol diet[28] while the crude extract of Moringa leaves has been reported to exhibit cholesterol lowering effect in high fat diet fed[8] and iron deficient rats[30] and in hyperlipedemics[31, 32].Numerous epidemiological studies suggest that herbs/diets rich in phytochemicals and antioxidants execute a protective role in health and disease[33]. Flavonoids, sterols, triterpenoids, alkaloids, saponins and phenolics are reported as bioactive antidiabetic principles[34, 35]. Flavonoids can regenerate damaged β-cells in the alloxan induced diabetic rats[36]. Polyphenols inhibit lipid peroxidation by acting as chain breaking peroxyl radical scavengers and can protect LDL from oxidation[37] and also inhibit hepatic lipid synthesis[38]. In this study, the plant extract was initially subjected to a preliminary phytochemical study and the results revealed the presence of Alkaloids, phenols, flavonoids, tannins and alkaloids as prominent bioactive components and some of the pharmacological activities of this plant may be attributed to the presence of these compounds. This conforms to the study of Sharma et al.[39] in which a hydro-ethanolic extract of the leaves of M.oleifera was revealed to possess various bioactive compounds of which alkaloids, flavonoids, flavonols, terpenoids, tannin and cardiac glycosides were prominent[39]. The DPPH assay is a widely used method for establishing the antioxidant activity of herbal extracts and phytochemicals. The ability to scavenge the DPPH radical is related to the inhibition of lipid peroxidation[40].The DPPH scavenging ability of this plant extract was found to be lower than that of Ascorbic acid but appreciably higher than that of BHT. The DPPH scavenging ability of EEMO may be attributed to the hydrogen donating ability of the extract and may serve as a free radical scavenger. Thus EEMO with its treasure of phytochemicals significantly ameliorated dyslipidemia and steatosis associated with NAFLD in experimental animals.The ameliorative mechanism of EEMO in NAFLD in this current study may be attributed to its antioxidant properties[41]. The presence of appreciable quantities of antioxidaive compounds in EEMO complements its nutritive role by normalising hepatic architecture in NAFLD[7]. Reactive oxygen species (ROS) are well known to play a significant role in the aetiology of insulin resistance, hyperglycemia and other pathological conditions that accompany NAFLD[42]. ROS can activate the nuclear factor κB (NF- κB) pathway[43, 44]. NF- κB is the main nuclear transcription factor that modulates the production of proinflammatory cytokines[45]. The flavonoids and other phenolics present in EEMO are potent chain breaking antioxidants[7]. Baumann et al.[46] reported that flavonoids can inhibit lipid peroxidation through their activity as strong scavengers of superoxide radical and singlet oxygen. The findings from this current study showed amelioration in hepatic oxidative stress by reducing the levels of malondialdehyde and modulating the activity of hepatic antioxidant enzyme SOD. This suggests that EEMO was able to counteract hepatic lipid peroxidation and oxidative stress. The high LD50 value of the plant is in accordance with other studies and this indicates its relatively high margin of safety[18].The ethanolic extract of Moringa oleifera leaves showed a great benefit in the improvement of NAFLD status via its antioxidant properties or by its indirect effects on insulin signaling and lipid profiles. However, the exact mechanisms with which these extracts improve the physiological status in NAFLD are not completely elucidated and would warrant further investigation.
ACKNOWLEDGEMENTS
- We appreciate the efforts of Professor Michael Aken’Ova of the University of Ibadan for his kind gestures. The Moringa leaves used in this study were obtained as a gift from his botanical garden. We would also like to thank Mr Duncan of the Department of Physiology, University of Lagos, Miss Best of the Department of Pharmaceutical Chemistry, University of Lagos, as well as Mr Adenekan and Mr Doherty of the Department of Biochemistry, University of Lagos, Lagos.
LIST OF ABBREVIATIONS
- ANOVA Analysis of VarianceBHT Butylated Hydroxy-TolueneCAT CatalaseCHOL CholesterolEEMO Ethanolic extract of Moringa oleiferaFBG Fasting blood glucoseGAE Gallic acid equivalentGSH Reduced GlutathioneH&E Haematoxylin and EosinHDL High density LipoproteinHDLC High density Lipoprotein CholesterolHFE High Fat EmulsionHOMA-IR Homeostasis model assessment for insulin resistanceIR Insulin resistanceLD50 Median Lethal doseLDL Low density LipoproteinLDLC Low density Lipoprotein CholesterolLSD Lowest significance differenceMDA MalondialdehydeMO Moringa oleiferaNAFLD Nonalcoholic Fatty Liver diseaseNASH Nonalcoholic SteatohepatitisPG PioglitazoneSOD Superoxide dismutaseTAG TriacylglycerolsTBA Thiobarbituric acidVLDL Very Low density LipoproteinVLDLC Very Low density Lipoprotein Cholesterol
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML