-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2013; 3(1): 1-5
doi:10.5923/j.ajb.20130301.01
Umbilical Cord Blood Plasmodium falciparum Infection on Alkaline Phosphatase Activity, Serum Liver Enzymes and Blood Chemistry of Neonates
Osaretin A. T. Ebuehi1, Oghenetega T. Oweh1, Olufunke M. Ebuehi2, Ayodeji A. Oluwole3
1Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria
2Department of Community Health & Primary Care, College of Medicine, University of Lagos, Lagos, Nigeria
3Department of Obstetrics and Gynaecology, College of Medicine, University of Lagos, Lagos, Nigeria
Correspondence to: Osaretin A. T. Ebuehi, Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
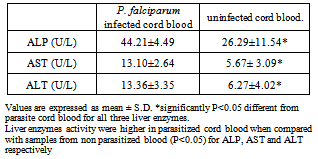
This study reports the activities of alkaline phosphatase (ALP), liver enzymes, nutrient markers and some biochemical indices in umbilical cord infected with and without P. falciparum.The activities of ALP, aspartate amino transferase (AST) and alanine amino transferase (ALT), as well as levels of serum urea, creatinine and triglyceride were assayed, The P. falciparum diagnosis was done using Giemsa stain technique.Results obtained show that ALP activity was significantly higher (P<0.05) in malaria infected cord blood (44.21±4.49) when compared with cord blood not infected by P. falciparum (26.29±11.54 U/l), that ALP has a relationship with Cord nutrients, a negative correlation with birth weight (r= - 0.07). Activities of liver enzymes (ALP, AST and ALT) were higher in P. falciparum infected cord blood (44.21±4.49, 13.10± 2.64 and 13.36±3.35 U/l) compared to non P. falciparum infected cord blood (26.29±11.54, 5.67±3.09 and 6.27±4.02 U/l). Results indicate an increase in ALP activity which bears a negative correlation with cord nutrients as well as birth weight. The increase in liver enzymes’ activities in P. falciparum infected cord could also indicate a compromise in hepatic integrity of the neonate.
Keywords: Cord Blood, Malaria, P. Falciparum, Alkaline Phosphatase Activity
Cite this paper: Osaretin A. T. Ebuehi, Oghenetega T. Oweh, Olufunke M. Ebuehi, Ayodeji A. Oluwole, Umbilical Cord Blood Plasmodium falciparum Infection on Alkaline Phosphatase Activity, Serum Liver Enzymes and Blood Chemistry of Neonates, American Journal of Biochemistry, Vol. 3 No. 1, 2013, pp. 1-5. doi: 10.5923/j.ajb.20130301.01.
Article Outline
1. Introduction
- Malaria is the most prevalent tropical disease in the world today. Each year, approximately 650 million people suffered from malaria, while between one and three million, mostly young children in Sub-Saharan Africa1. Nigeria is known for high prevalence of malaria and it is a leading cause of morbidity and mortality in the country. Available records show that at least 50 per cent of the population of Nigeria suffers from at least one episode of malaria each year and this accounts for over 45 per cent of all out- patient visits2.Malaria infection during pregnancy is a major public health problem in tropical and subtropical regions throughout the world3. Malaria during pregnancy has been most widely evaluated in Africa South of the Sahara, where 90% of the global malaria pregnant women who came for their ante-natal visit at burden occurs. The burden of malaria infection during pregnancy is caused mainly by Plasmodium falciparum, the most common malaria species in Africa4,5. Every year at least 3 million pregnancies occur among women in malarious areas of Africa, most of whom reside in areas of relatively stable malaria transmission6. Pregnant women and the unborn children are vulnerable to malaria, which is a major cause of peri- natal mortality, low birth weight and maternal anaemia7.Cord blood is a sample of blood taken from a newborn baby's umbilical cord. Umbilical Cord Blood also known as cord blood is the blood that remains in the placenta and in the attached umbilical cord after child birth 1,3,6,8. Cord blood is collected because it contains stem cells that can be used to treat hematopoietic and genetic disorders. It is collected by syringing out the placenta through the umbilical cord at the time of child birth after the cord has been detached from the newborn.Alkaline phosphatase is present in all tissues throughout the human body but it is particularly concentrated in liver, bile duct, kidney, bone and placenta. Placenta alkaline phosphatase (PALP) is synthesized in the placenta syncytiotrophoblast, from the 12th week of pregnancy and is probably involved in trans-placenta immunoglobulin G (lgG) transport, as well as nutrient transport from parents to offsprings8. Increased synthesis of placenta alkaline phosphatase in cord blood has been observed to correspond to the nutritional demand of foetus during growth.Some conditions have been reported to affect alkaline phosphatase activity which plays a major role in nutrient transfer from placental to umbilical cord. Heavy metals such as cadium have been reported to reduce alkaline phosphatase activity as well as drugs, such as oral contraceptive. The effects of malaria parasite (P. falciparum) transmitted by the female anopheles mosquito on alkaline phosphatase level in cord blood as well as how this affects liver enzymes of the newborn is yet to be reported. This study is therefore designed to investigate the effect of cord blood P. falciparum infection on alkaline phosphatase and liver enzymes activities and blood chemistry of the neonate.
2. Subjects and Methods
2.1. Study Design and Subjects
- This study is a cross-sectional study that involve sample collection from the Lagos University Teaching Hospital (LUTH) from 53 patients who have live birth (either normal delivery or via C/S). Women who were HIV positive or had sexually transmitted infections at booking, those with pre-term deliveries, as well as those with multiple pregnancies or with chronic diseases, such as hypertension and diabetes were excluded. Ethical approval was obtained from the Health Research and Ethical Committee, Lagos University Teaching Hospital (LUTH). Fifty three cases of uncomplicated and safe, consenting pregnant women in apparent good health were used for this study. These 53 cases were collected from Lagos University Teaching Hospital (LUTH) and the subjects’ age range between 24 and 44 years. All the participants in this study gave their informed consent.
2.2. Collection of Blood Samples
- Blood samples were collected from umbilical cord vein into EDTA, flouride oxalate and lithium heparin bottles. The samples collected in the fluoride oxalate and lithium heparin bottles are centrifuged at 1200rpm for 5min at room temperature (28-31°C). The supernatant from the lithium heparin bottle was divided into two portions and stored frozen in bijou bottles until needed for analysis, which was done within 48 h. Blood sample in the EDTA bottle are used for parasitological examination. The mother ages, weight and sex of the newborn were recorded.
2.3. Parasitological Examination
- Blood samples collected with EDTA container were diagnosed for malaria by microscopy.Thick and thin blood films were prepared immediately upon blood collection on the same slide. For thick film, 12 microlitre of blood was spread over a diameter of 15mm, while 2 microlitre of blood was used for thin films. The thin film was then fixed in absolute methanol for 1-2 second and air-dried. The blood films were stained after 24-48 hours with 3% Geimsa stain solution at pH 7.2. After which the slides were viewed under the microscope and malarial parasites counted per high power field and the density was graded as follows: 1 parasite/field: Low density (+), 2-9 parasites / field: medium density (++), >20 parasites/field: high density (+++) 9.
2.4. Assay of Liver Enzymes
- The activity of alanine amino transferase also known as glutamate pyruvate transaminase (SGPT) and aspartate amino transferase also known as glutamate oxaloacetate transaminase (SGOT), as well as alkaline phosphatase were estimated using standard colorimetric diagnostic kits (Randox Laboratories Limited, England) by the method of Reitman and Frankel10 and Wright et al.,11.
2.5. Blood Chemistry Assay
- The determination of the concentrations for serum triglyceride, urea and creatinine were carried out using colorimetric diagnostic kits (Randox Laboratories Limited, England) according to the method of Trinder12, Veniamin and Vakirtzi-Lemonias13 and Tietz et al.,14 respectively.
2.6. Statistical Analysis
- Data of the study were expressed as mean ±standard deviation and further analysed using the SPSS version 20.0. A P- value of P<0.05 was used as indicator of statistical significance.
3. Results
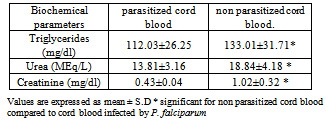
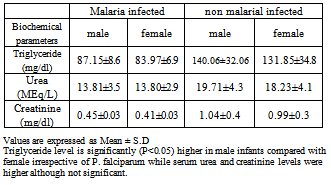
- The effects of cord blood p. falciparum infection on alkaline phosphatase activity, serum liver enzymes and blood chemistry of neonates was investigated and results are presented in Tables 1-6.
|
|
|
|
|
|
4. Discussion
- Data of the study indicate significant increase (P<0.05) in serum alkaline phosphatase activity in malarial infected cord blood when compared with cord blood not infected by malaria. However, the degree of parasiteamia did not significantly alter serum alkaline phosphatase (ALP) activity. The increase in ALP in parasitized cord blood could be as a result of the defensive role ALP offers to the umbilical cord, helping it fight against infection from the placenta and toxic materials15. ALP increases following polymorphonucleated (PMN) cells activation, e.g. during infections16 or after stimulation by granulocyte colony-stimulating factor 17. It is therefore possible that PMN have a role in defence against malaria18. This could also explain the mechanism of ALP as a defence in the umbilical cord. There is paucity of information on alkaline phosphatase activity in cord blood. The increased ALP activity in malarial infected cord blood allows for nutrients to still be transferred as a normal ALP activity in parasitized cord blood will hamper the transfer of nutrients from placenta to cord blood. The ALP activity was not significantly altered by the degree of parasiteamia and the present findings concur with the previous report 19.Other results obtained showed the effect of P. falciparum on Liver enzymes and also the degree of parasiteamia in relation to liver enzymes activity in cord blood. The activities of liver enzymes (ALP, AST,ALT) of P. falciparum infected cord blood were significantly higher compared activities of liver enzymes of cord blood not infected (P< 0.05). This observation could demonstrate that the hepatic stage of the parasite life cycle in its human host is accompanied by significant pertubation in the hepatocyte membrane leading to leakage of the liver enzymes into a general circulation. The possible mechanism could be due to the fact that when the liver cells are invaded by the sporozoite form of the malarial parasites, it can cause organ congestion, sinusoidal blockage and cellular inflammation20. These changes in hepatocytes can lead to the leakage of parenchymal (transaminases) and membraneous (alkaline phosphatase) enzymes of the liver into the circulatory system21 hence the increase in liver enzymes which have also been observed among malarial infected patients as reported previously22. It was also demonstrated that the various liver enzyme activities in serum increased with increase in malarial parasite density 23. This report corresponds also with a study which reported that P. falciparum infection significantly increase the activity of liver enzymes. The increase in liver enzymes of babies whose cord blood is infected by P. falciparum could be an early marker for liver dysfunction and could compromise membrane integrity. However, high ALP activity is not a useful marker for liver dysfunction in newborn, because of their bone formation process. Also the degree of parasiteamia correlates positively with liver enzymes of cord blood with the 2+ degree of parasiteamia showing higher values of all liver enzymes when compared to 1+ , but it was not statistically significant. Results from this study report the effect of P. falciparum infection on blood chemistry of the cord blood of male and female babies. Serum urea and creatinine are biochemical markers for renal dysfunction, and its early diagnosis could be useful in providing information concerning the integrity of the kidney. The serum triglyceride, urea and creatinine were significantly (P>0.05) higher in cord blood samples not infected with P. falciparum when compared to cord blood infected by P. falciparum. This corresponds with previous report29 which reported a high serum creatinine level in normal neonate particularly in the first 48 hours after birth and a subsequent reduction in creatinine level after day 2. Serum triglyceride, urea and creatinine levels were higher in male cord blood samples compared to the female counterpart in both parasitized and non parasitized cord blood and this difference was only statistically significant(P<0.05) in triglyceride level.Data from the study also compare the mothers parameters and that of the child. Form the result, serum levels of glucose and albumin, ALT activity were higher in the mother blood as compared to those of umbilical cord blood, although were not statistically significant (P>0.05). Other biochemical parameters, such as serum cholesterol, triglyceride, total protein, ALP, AST, urea, creatinine levels , AST and ALP activities were slightly higher than in the cord blood when compared to their respective serum levels of the respective mothers, with no statistical significance (P>0.05). This could possibly be due to the fact that these biochemical parameters in the cord blood are transferred from the placenta of the mothers.Malaria has been reported to be a cause of high infant mortality24,25,26,27,28, although no clear mechanism has been demonstrated as to how this causes death of infants. Evidence from this study of low birth weight as well as high activities of liver enzymes in newborn whose cord blood are infected with P. falciparum, compromising hepatic function, could be a pointer to infant mortality caused by malaria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML