-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2012; 2(6): 98-103
doi: 10.5923/j.ajb.20120206.02
Food Value and Phytochemical Composition of Luffa cylindrica Seed Flour
Oyetayo Folake Lucy , Ojo Babajide Abidemi
Department of Biochemistry, Ekiti State University Ado–Ekiti, P.M.B. 5363, Ado-Ekiti, Ekiti State, Nigeria
Correspondence to: Oyetayo Folake Lucy , Department of Biochemistry, Ekiti State University Ado–Ekiti, P.M.B. 5363, Ado-Ekiti, Ekiti State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
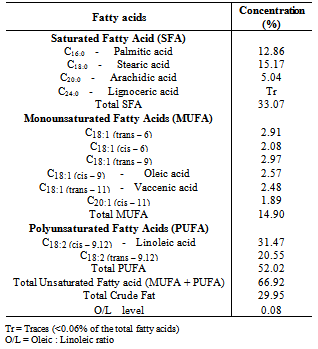
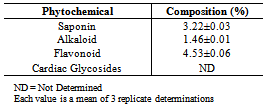
Amino acid, fatty acid and phytochemical compositions of Luffa cylindrica seed flour were investigated. The total amino acid concentration was 72.71g/100g protein and the total essential amino acid concentration was 38.76g/100g protein. Arginine (9.75g/100g protein) was the most concentrated essential amino acid while the Lysine/Arginine ratio was 0.52. The calculated isoelctric point (pI), predicted protein efficiency ratio (P-PER) and limiting amino acid were 5.91, 1.89 and threonine respectively. Linoleic acid (31.47%) was the most abundant fatty acid in the seed flour. Unsaturated fatty acids (66.9%) were of higher concentration than saturated fatty acids (33.07%) while the total concentration of polyunsaturated fatty acids was 52.02%. Flavonoids (4.53%) were the most concentrated phytochemical in the Luffa cylindrica seed flour while saponins, alkaloids and cardiac glycosides were also present. This study shows that Luffa cylindrica seed contains a high proportion of essential amino acids and has a low atherogenic potential. Also, it is a potential source of healthy fat that may be useful as source of food nutrient and phytomedicine.
Keywords: Amino Acid, Fatty Acid, Phytochemical Composition, Luffa Cylindrica, Lysine/Arginine Ratio, Polyunsaturated Fatty Acids, P-PER
Cite this paper: Oyetayo Folake Lucy , Ojo Babajide Abidemi , "Food Value and Phytochemical Composition of Luffa cylindrica Seed Flour", American Journal of Biochemistry, Vol. 2 No. 6, 2012, pp. 98-103. doi: 10.5923/j.ajb.20120206.02.
Article Outline
1. Introduction
- Luffa cylindrica (smooth luffa), belongs to the plant family Cucurbitaceae. It is a herbaceous plant and thrives commonly with twining tendrils[1]. It is a large succulent tendril climber with slender, slightly hairy furrowed stem. The interior is cucumber-like when immature, but quickly develops into a network of fibre surrounding large number of flat blackish seeds. The plant is reported to have originated from India but grows predominantly as weed in most parts of Nigeria. The matured fruits are used for domestic purposes as sponge. It is an excellent fruit in nature containing all the essential constituents required for good health of humans[2]. Its kernel contains between 45 – 51% oil which is composed of mainly oleic and linoleic acids. The seeds have laxative properties due to their high oil content. It contains a wide range of secondary metabolites with distinct biological activities. The seeds have been reported to be useful in the treatment of asthma, sinusitis and fever[3]. It is reported to possess antiviral, anti-tumor, antioxidant, anti-inflammatory and immunomodulatory activities[4]. Up till date, there has been no report of toxicity, adverse effects or drug interactions associated with consumption of luffa. There are limited information on the chemical composition of Luffa cylindrica seeds. Therefore, this study aims at investigating the potentials of the Luffa cylindrical seed flour as an additional source of nutrients and phytochemicals in human and animal diets in the developing world.
2. Materials and Methods
2.1. Sample Collection
- Luffa cylindrica fruits were collected from Ado Ekiti main market, Ado-Ekiti, Nigeria. They were identified at the Department of Plant Science, University of Ado-Ekiti, Nigeria. The seeds were removed from the fibrous interior and washed with water. The seeds were dehulled, oven-dried at 60℃ for 5 hours and pulverized using a Panasonic Electronic blender. The powdered seeds were kept in a container prior to analysis.
2.2. Amino Acid Analysis
- The amino acid profile was determined using methods described by Spackman et al[5]. 0.2g of powdered seed sample was defatted, hydrolyzed, evaporated in a rotary evaporator and loaded into the automatic amino acid analyzer, Technicon Sequential Multi-Sample (TSM) analyzer. The sample was hydrolyzed for determination of all amino acids except tryptophan in consistent boiling hydrochloric acid for 22 hours under a nitrogen flush.
2.3. Fatty Acid Analysis
- The crude fat analysis was carried out by following the ether extraction method of AOAC[6]. Fat was extracted in a Soxhlet apparatus using petroleum ether. Fatty acid methyl esters (FAME) were prepared by boron trifluoride-catalyzed transesterification, according to AOAC method[6]. Methyl heptadecanoate was used as the internal standard. FAME were then analyzed on a Fisons 8000 series gas chromatography equipped with a hydrogen flame ionization detector (FID). Separation was performed using a fused silica capillary column (100m x 0.25mm i.d.), coated with 0.20µm SP2560 (Supelco Inc., Bellefonte, PA) as the stationary phase. The oven temperature was programmed at 140℃ for 5min, and then ranged from 140℃ to 240℃ at a rate of 4℃ per min. The injector and FID were at 260℃ at a rate of 4℃ per min. A reference standard quantitative FAMEs mix (Supelco Inc.) was analyzed under the same operating conditions to determine the peak response factor, for identification and quantification of the individual fatty acids.
2.4. Phytochemical Screening and Quantitative Analysis
- The Luffa cylindrica seed flour were screened for the presence of saponin, flavonoid, Alkaloid and Cardiac glycoside prior to quantitative analysis.
2.5. Test for Saponin
- The ability of saponins to produce frothing in aqueous solution and to haemolyse red blood cells was used to screen these compounds. 1.0g of the sample extract was shaken with distilled water in a test tube. Frothing persisted on warming, giving an evidence for the presence of saponin[7].
2.6. Test for Alkaloids
- 0.2g of the extract from the sample was stirred in 5ml of 1% aqueous hydrochloric acid in a steam bath. 1ml of the filtrate was treated with a few drops of Dragendorff’s reagent. Turbidity or precipitation was taken as the preliminary evidence for the presence of alkaloids in the sample extract being evaluated[8].
2.7. Test for Flavonoids
- 2g of the sample was heated with 10ml of ethyl acetate over a steam bath for 3 minutes. The mixture was filtered and 4ml of the filtrate was shaken with 1ml of dilute ammonia solution. A yellow colouration was observed indicating a positive test for flavonoids[9].
2.8. Test for Cardiac Glycosides
- 0.5g of dried extract was dissolved in 2.0ml of glacial acetic acid containing one drop of ferric chloride solution. This was then underlaid with 1.0ml of concentrated H2SO4. A brown ring at the interface indicated the presence of glycosides.
2.9. Saponin Concentration Determination
- 100cm3 of 20% aqueous ethanol was added to 20g of the sample in a conical flask and heated at 55℃ over a hot water bath for 4 hours with continuous stirring. The mixture was filtered and residue re-extracted with 200ml 20% ethanol. The combined extracts were reduced to 40ml over a water bath at 90℃. 20ml of diethyl ether was added to the concentrate in a 250ml separating funnel and shaken vigourously. The aqueous layer was recovered and purification process repeated. 60ml of n-butanol was added to the extract and then washed twice with 10ml of 5% aqueous NaCl. The solution was evaporated in a water bath. The sample was oven-dried to a constant weight and saponin content calculated as percentage[10].
2.10. Flavonoid Concentration Determination
- 100ml of 80% aqueous methanol was used to extract 10g of sample repeatedly at room temperature and filtered through Whatman filter paper No 42 (125mm). The filtrate was evaporated to dryness in a crucible over a water bath until a constant weight was reached[11].
2.11. Alkaloid Concentration Determination
- 200ml of 10% acetic acid in ethanol was added to 5g of sample in a 250ml beaker, it was covered and allowed to stand for 4 hours. Filtration followed and the extract was concentrated on a water bath to one quarter the initial volume. Concentrated NH4OH was added drop wise to the extract until precipitation was complete. The whole solution was allowed to settle and precipitate was collected and washed with dilute NH4OH and then filtered. The residue is the alkaloid which was dried and weighed[9].
3. Discussion and Conclusions
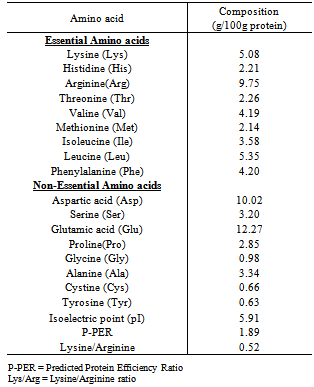
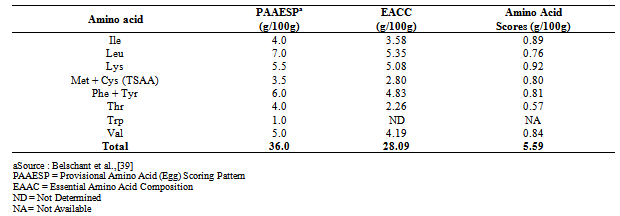
- Table 1 shows the amino acid profile of Luffa cylindrica seed flour. The most concentrated amino acid in the seed flour was glutamic acid (12.27g/100g) while arginine (9.75g/100g) was the most concentrated essential amino acid. Aremu et al.[12] made a similar observation (5.1g/100g) in L. cylindrica seed kernel. Arginine is an essential amino acid which has direct antioxidant activity[13] and also necessary for children growth[14]. The methionine content in the sample (2.14g/100g) is found to be higher than that of soyabean (0.9g/100g)[15] while the lysine content (5.08g/100g) is higher than that reported by Aremu et al.[12] for L. cylindrica seed kernel (2.9g/100g) and Macaiba Palm kernel protein (3.85g/100g)[16]. The concentrations of isoleucine (3.58g/100g) and valine (4.19g/100g) were found to meet the FAO/WHO/UNU[17] requirement for these two essential amino acids (2.8g/100g and 3.5g/100g respectively) for Pre-school children aged 2-5 years. Predicted Protein Efficiency ratio (P-PER) value is one of the quality parameters used in the evaluation of proteins[18]. P-PER value for L. cylindrica seed protein was 1.89. This is higher than 1.03 obtained by Salunkhe and Kadam[19] for Grass Pea (Lathyrus sativus). The calculated isoelectric point (pI) is of significance in protein purification. Luffa cylindrica seed flour had a calculated pI of 5.91 which is the point at which the protein should accumulate during purification. The Lysine/Arginine (Lys/Arg) ratio has been reported to play a role in the artherogenocity of a protein. Kritchevsky[20] reported that a Lys/Arg ratio as high as 2.0, increases the artherogenic potential of a diet. The Lys/Arg ratio of the seed flour (0.52) is much more lower than that of casein with Lys/Arg ratio 2.0. A similar observation has been made for Kerstingiella geocarpa[21]. Thus, since L. cylindrica has a low Lys/Arg ratio, it will possess a low artherogenic potential.
|
|
|
|
|
References
| [1] | Ajiwe, V.I.E. – Ndukwe, G.I. – Anyadiegwu, I.E.: Vegetable diesel fuels from Luffa cylindrica oil, its methylester and ester-diesel blends. ChemClass Journal, 2, 2005, pp.1 – 4 |
| [2] | Rahman, A.S.H.: Bottle gourd (Lagenaria siceraria) : a vegetable for good health. Natural Product Radiance, 2, 2003, pp.249 – 250 |
| [3] | Nagao, T. – Lanaka, R. – Iwase, Y. – Hanazone, H. – Okabe, H.: Studies on the constituents of Luffa acutangula. Roxb. Clinical Pharmacology Bulletin, 39, 1991, pp.599-606 |
| [4] | Tannin-Spitz, T. – Bergman, M. – Grossman, S. Cucurbitacin glucosides : Antioxidant and free-radical scavenging activities. Biochemical and Biophysical Research Communications, 364, 2007, pp.181 – 186 |
| [5] | Spackman, D.H. – Stein, E.H. – Moore, S.: Automatic Recording Apparatus for use in the chromatography of amino acids. Analytical Chemistry, 30, 1958, pp.1191 |
| [6] | AOAC.: Official Method of Analysis. Association of Official Analytical Chemists, 16th Ed. Washington DC, USA. AOAC, 1998 |
| [7] | Cuilei, I.: Methodology for analysis of practical manual on the industrial utilization of medicinal and aromatic plants. Center of the UNIDO Romantia 1982, pp.67 |
| [8] | Gundidza, M.: Phytochemical screening of some Zimbabwean Medicinal Plants. The Central African Journal of Medicine , 31, 1985, pp.238 |
| [9] | Harborne, J.B.: Phytochemical Methods : A guide to modern techniques of plant analysis. 1st ed. London: Chapman & Hall Ltd., 1973. 278pp. ISBN 0-4121-0540-3 |
| [10] | Obadoni, B.O. – Ochuko, P.O.: Phytochemical studies and comparative efficacy of the crude extracts of some Homostatic plants in Edo and Delta States of Nigeria. Global Journal of Pure and Applied Science, 8b, 2001, pp. 203-208 |
| [11] | Boham, B.A. – Kocipal-Abyazan, R.: Flavonoids and condensed tannins from leaves of Hawaiian vaccinium vaticulatum and V. calycinium. Pacific Science, 48, 1974, pp. 458 – 463 |
| [12] | Aremu, M.O. – Olaofe, O. – Okiribiti, B.Y.: Chemical Evaluation of the Nutritive value of smooth luffa (Luffa cylindrica) seed’s kernel. Electronic Journal of Environmental and Agricultural Food Chemistry, 7(10), 2008, pp.3444 – 3452 |
| [13] | Boger, R.H – Bode-Boger, S.M - Mugge, A. – Klenke, S – Brandes, R – Dwenger, A. – Frollich, J.C.: Supplementation of hypercholesterolemic rabbits with L-Arg reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis, 117, 1996, pp273 - 284 |
| [14] | Robinson, D.E.: Food Biochemistry and Nutritional Value. Longman Scientific and Technology, Burnmell, Haslow, England, 1987, p327 – 328 |
| [15] | Akintayo, E.T. – Adebayo, E.A. – Arogundade, L.A.: Chemical composition, physicochemical and functional properties of akee (Bilphia sapida) pulp and seed. Food Chemistry, 77, 2002, pp333 – 336 |
| [16] | Bora, P.S. – Rocha, R.V.M.: Macaiba Palm : Fatty and Amino acids composition of fruits. ALTAGA , 4(3), 2004 , pp.158 – 162 |
| [17] | FAO/WHO/UNU: Energy and Protein requirements. Technical report series, Geneva, 275, 1985, pp.204 |
| [18] | FAO/WHO: Protein quality evaluation report of joint FAO/WHO expert consultative FAO. Food and Nutrient, 1991 |
| [19] | Salunkhe, D.K. – Kadam, S.S.: Handbook of World Food Legumes, Nutritional Chemistry, Processing Technology and Utilization. Boca Raton, F.L. (USA) CRC Press, 1, 1989, ISBN: 0849305543 |
| [20] | Kritchevsky, D.: Vegetable protein and atherosclerosis. Journal of American Oil Chemical Society, 56, 1979, pp.135 – 140 |
| [21] | Ajayi, O.B. – Oyetayo, F.L.: Potentials of Kerstingiella geocarpa as a health food. Journal of Medicinal Food, 12(1), 2009, pp.184 - 187 |
| [22] | Pellet, P.L. – Young, V.R.: Nutritional evaluation of protein foods, Report of a working group sponsored by the International Union of Nutritional Sciences and the United Nations University World Hunger Programme. The United Nations University, 1980, pp167 |
| [23] | Aremu, M.O. – Atolaye, B.O. – Pennap, G.R. – Ashikaa, B.T.: Proximate and amino acid composition of mesquite bean (Prosopis africana) protein concentrate. Indian Journal of Botany Research, 3(1), 2007, pp.114 - 119 |
| [24] | Oshodi, A.A. – Esuoso, K.O. – Akintayo, E.T.: Proximate and amino acid composition of some under-utilized Nigerian legume flour and protein concentrates. La Rivista Italiana Delle Sostanze Grasse, 75, 1998, pp. 409–412 |
| [25] | Olaofe, O. – Adeyemi, F.O. – Adeniran, G.O.: Amino acid, mineral composition and functional properties of some oil seeds. Journal of Agric and Food Chemistry, 42(4), 1994, pp.879 – 881 |
| [26] | Ogungbenle, H.N.: Chemical composition, functional properties and amino acid composition of some edible seeds. La Rivista Italiana Delle Sostanze Marzo, 83, 2006, pp.81 – 86 |
| [27] | Garret, R.H. – Grisham, C.M.: Lipids In Biochemistry. Thomson Brooks/Cole 3rd Ed., 2005, pp.247 – 266. |
| [28] | Kinsella, J.E.: Possible mechanisms underlying the effects of n3 polyunsaturated fatty acids. Omega 3 News, 1-5, 1990. |
| [29] | Willet, W.C.: The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. Journal of Cardiovascular Medicine (Hagerstown), 8(Suppl 1), 2007, pp.42 – 45 |
| [30] | Branch, W.D. – Nakayama, T. – Chinnan, M.S.: Fatty acid variation among US runner type peanut cultivars. Journal of the American Oil Chemist’s Society, 67, 1990, pp.591 – 596 |
| [31] | Schneider, G. – Wolfling, J.: Synthetic Cardenolides and related compounds. Current Organic Chemistry, 8(14), 2004, pp.1381 – 1403 |
| [32] | Okwu, D.E. – Omodamiro, O.D.: Effects of hexane extract and phytochemical content of Xylopia aethiopica and Ocimum gratissimum on the uterus of guinea pig. Bioresearch, 3(2), 2005, pp.40 – 44 |
| [33] | Edeoga, H.O. – Okwu, D.E. – Mbaebie, B.O.: Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology, 4(7), 2005, pp.685-688 |
| [34] | Bagchi, M. – Mark, M. – Casey, W. – Jaya, B. - Xumei Y. – Sidney, S. – Debasis, B.: Acute and Chronic stress-induced oxidative gastrointestinal injury in rats, and the protective ability of a novel grape seed proanthocyanidin extract. Nutrition Research 19, 1999, pp.1189 – 1199 |
| [35] | Shoskes, D.A. – Zeitlin, S.I. – Shahed, A. – Rajfer, J.: Quercetin in Men with category III chronic prostatitis: A preliminary prospective, double – blind, placebo – controlled trial. Urology, 54(6), 1999, pp.1001 – 1201 |
| [36] | Cushnie, T.P.T. – Lamb, A.J.: Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26(5), 2005, pp.343 – 356 |
| [37] | Oakenfull, D. – Sidhu, G.S.: Could Saponins be a useful treatment for hypocholesterolemia? European Journal of Clinical Nutrition, 44, 1990, pp.79 – 88 |
| [38] | Amakoha, R.A. – Ubwa, S. – Otokpa, T. – Shenge, G.: Phytochemical composition and biological activities of Uvaria chamae. Urology, 54(6), 2002, pp1001- 1201 |
| [39] | Belschant, A.A. – Lyon, C.K. – Kohler, G.O.: In Food Protein Sources (N.W. Pirieed). Cambridge University Press, Cambridge, UK, 1975 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML