-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2012; 2(6): 94-97
doi: 10.5923/j.ajb.20120206.01
NutriSim© Diminishes an Endotoxin-stimulated Proinflammatory Cytokine Production
Erika D. G. Renovato 1, Rolando R. Davalos 2, Luis A. R. Tirado 2, Jose J. H. Andalon 2, Irma E. V. Brizuela 3, Jose A. C. Ramos 4, Erandis D. T. Sanchez 4, Genaro G. Ortiz 4, Fermin P. P. Moises 1
1Departamento de Química, Centro Universitario de Ciencias Exactas e Ingenierías, Universidad de Guadalajara, Guadalajara, Jalisco, México
2Área de Desarrollo e Investigación Científica, Laboratorios BioSim©, D.F, México, México
3OPD Instituto Jalisciense de Cancerología, Guadalajara, Jalisco, México
4Laboratorio de Mitocondria, Estrés Oxidativo & Patología, División de Neurociencias, Centro de Investigación Biomédica de Occidente, Instituto Mexicano del Seguro Social, Guadalajara, Jalisco, México
Correspondence to: Fermin P. P. Moises , Departamento de Química, Centro Universitario de Ciencias Exactas e Ingenierías, Universidad de Guadalajara, Guadalajara, Jalisco, México.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Lipopolysaccharide, a main component of Gram-negative bacterial endotoxin is the leading cause of sepsis or endotoxic shock. This condition generates tissue hypoperfusion and leads to a state of acute metabolic-circulatory dysfunction and multiorgan systemic failure which contributes to amplify the inflammatory response and resulting in the release of pro-inflammatory cytokines (TNF-α, IL-1 and IL-6). The effect of NutriSim© on serum pro-inflammatory cytokine concentrations were investigated in lipopolysaccharide-treated male Wistar rats. Animals were randomized into five groups: control groups receiving a single intraperitoneal injection of physiologic saline solution or NutriSim© (10µl), respectively; LPS (20 mg/Kg, i.p.); NutriSim© treatment 15 min before or after LPS in the same aforementioned dose. The results showed that a single dose of NutriSim© prior or before the endotoxic insult diminishes significantly the production of serum TNF-α, IL-1 and IL-6. Therefore NutriSim© can modulate an inflammatory response in experimental model of endotoxic shock.
Keywords: Nutrisim©, TNF-α, IL-1, IL-6, Endotoxic Shock, Lipopolysaccharide
Cite this paper: Erika D. G. Renovato , Rolando R. Davalos , Luis A. R. Tirado , Jose J. H. Andalon , Irma E. V. Brizuela , Jose A. C. Ramos , Erandis D. T. Sanchez , Genaro G. Ortiz , Fermin P. P. Moises , "NutriSim© Diminishes an Endotoxin-stimulated Proinflammatory Cytokine Production", American Journal of Biochemistry, Vol. 2 No. 6, 2012, pp. 94-97. doi: 10.5923/j.ajb.20120206.01.
Article Outline
1. Introduction
- Endotoxic shock is a systemic response to a bacterial infection caused by endotoxins or lipopolysaccharide (LPS)[1]. LPS is a complex glycolipid, composed of a hydrophilic polysaccharide moiety and a hydrophobic domain known as lipid A, this is the toxic region of the molecule, and responsible for induction ofimmunostimulatory properties of LPS[2]. LPS is a major component of the outer membrane of Gram-negative (GN) bacteria such as Escherichia coli, and one of the most potent microbial initiators of inflammation[3−5].The inflammatory response to LPS is crucial for protecting the host against pathogenic bacteria, nevertheless uncontrolled and excessive production of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), and interleukin (IL)-1, IL-6, IL-8, and IL-12[6] can be harmful. Cytokines and other mediators may lead to serious systemic complications including microcirculatory dysfunction, liver and kidney damage[7,8], and septic shock with a high mortality[9].NutriSim©, a nutritive supplement that contains arginine, lysine, choline, ammonium chloride, calcium and magnesium is used empirically in the treatment of several degenerative disorders[10]. Previously, we have shown that NutriSim© protects against brain damage induced by ischemia-reperfusion in Mongolian gerbils. These effects are partly attributed to its antioxidant action[11]. Free radicals are thought to be involved in the inflammatory process[12], therefore in order to keep evaluating the potential of NutriSim©, the current study screened anti-inflammatory effect of that nutritive supplement onlipopolysaccharide-induced cytokines production.
2. Materials and Methods
2.1. Materials (Reagents)
- The lipopolysaccharide (LPS) of Escherichia coli, serotype 0111:B4 was purchased from Sigma Chemical Co. (St. Louis, MO, USA), enzyme-linked immunosorbent assays (ELISA) Kits for cytokine determination specific for rat, was obtained from R&D Systems (Minneapolis, MN, USA) and NutriSim© was acquired from Laboratorios BioSim© (D.F, México).
2.2. Animals
- Sixty adult male Wistar rats used for this research work were obtained from CIBO-IMSS (Guadalajara, Jalisco, Mexico). The animals weighed 200 - 250 g, maintained on a 12h/12h light-dark cycle at (22 ± 2℃), fed with standard diet (Chow Purina) and water ad libitum.
2.3. Experimental Design
- Animals were randomly assigned to five experimental groups as shown below. Group Control; treated with physiologic saline solution administered by intraperitoneal (i.p.) injectionGroup NutriSim©; received a single dose (10 µl/100g of body weight, i.p.) of NutriSim©.Group LPS; received a LD100 dose of LPS (20mg/Kg of body weight, i.p.).Group LPS plus NutriSim©; received a LD100 dose of LPS (20mg/Kg of body weight, i.p.), 15 min before NutriSim©. Group NutriSim© plus LPS; NutriSim© was administered with a dose of NutriSim© (10 µl/100g of body weight, i.p.) 15 min before LPS.Animals were sacrificed at 15, 30 and 60 min after the treatment; blood was collected immediately and centrifuged for 10 minutes, at 3000 rpm for collection of serum. Protein concentration was determined by the Bradford method using bovine serum albumin as a standard[13].
2.4. Cytokines Quantifications (ELISA)
- TNF-
 , IL-1β and IL-6 levels in the serum were quantified using commercially available ELISA kits specific for rat TNF-α (Genzyme, Cambridge, MA, USA), IL-1β and IL-6 (both from Endogen, Woburn, MA, USA). The sensitivity of the assays for TNF-α, IL-1β and IL-6 was 5 pg/ml, 12 pg/ml and 15 pg/ml, respectively. Absorbance at 405-414 nm was measured at a maximum of 15 min after the addition of substrate, using a UV-Vis spectrophotometer (BIO-RAD 550).
, IL-1β and IL-6 levels in the serum were quantified using commercially available ELISA kits specific for rat TNF-α (Genzyme, Cambridge, MA, USA), IL-1β and IL-6 (both from Endogen, Woburn, MA, USA). The sensitivity of the assays for TNF-α, IL-1β and IL-6 was 5 pg/ml, 12 pg/ml and 15 pg/ml, respectively. Absorbance at 405-414 nm was measured at a maximum of 15 min after the addition of substrate, using a UV-Vis spectrophotometer (BIO-RAD 550). 2.5. Statistical Analysis
- Statistical significance of differences among groups was tested by one-way analysis of variance (ANOVA), followed by multiple comparisons between each group and control or other groups using Newman-Keuls t test; p ≤ 0.05 was considered significant.
3. Results
3.1. Serum Cytokines Levels
- Basal levels of serum TNF-α, IL-1β and IL-6 were detected in saline-treated rats, respectively. Pre-treatment of rats with NutriSim© alone did not affect the serum level of these cytokines (Figures 1-3). The concentration of TNF-α in serum rapidly increased, reaching a peak at 30 min after injection of LPS, then diminishes. This increase was found to be statistically significant in comparison with the control group (p<0.01). NutriSim© prevents excessive expression of TNF-α in response to LPS (p<0.01), however the effect was higher when injected prior LPS administration (Figure 1).
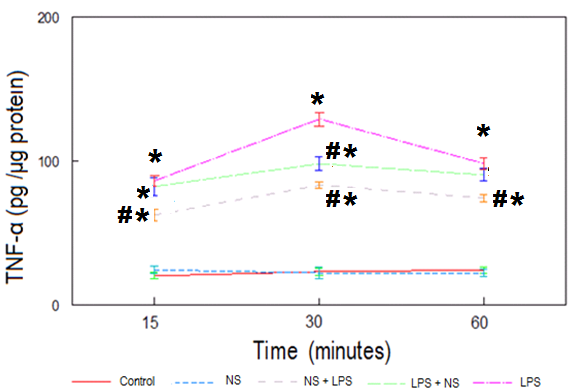 | Figure 1. Time course of serum TNF- levels from the indicated rat groups. Each point represents the mean ± SEM. *P<0.01 vs. control groups. # P<0.01 vs. LPS group levels from the indicated rat groups. Each point represents the mean ± SEM. *P<0.01 vs. control groups. # P<0.01 vs. LPS group |
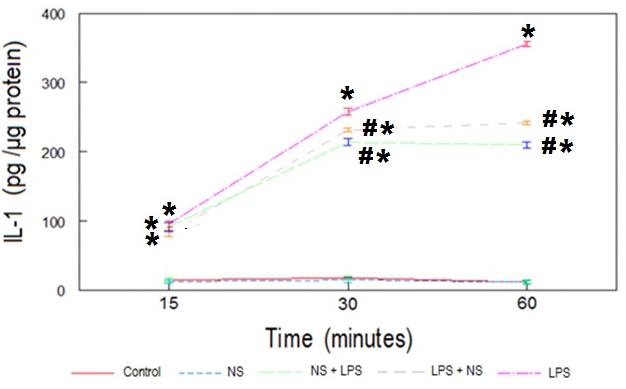 | Figure 2. Time course of serum IL-1β levels from the indicated rat groups. Each point represents the mean ± SEM. *P<0.01 vs. control groups. # P<0.01 vs. LPS group |
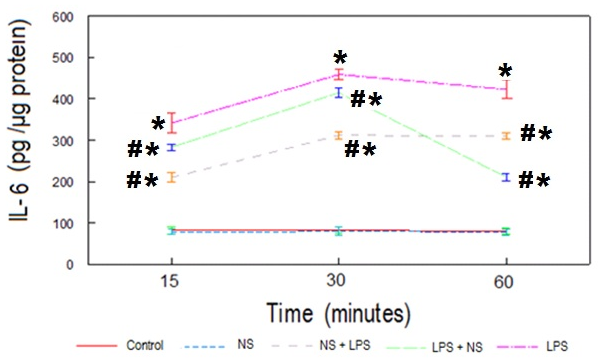 | Figure 3. Time course of serum IL-6 levels from the indicated rat groups. Each point represents the mean ± SEM. *P<0.01 vs. control groups. # P<0.01 vs. LPS group |
4. Discussion
- We found that intraperitoneal administration of lipopolysaccharide evoked a robust systemic response that induced a stereotypical cytokine release. Macrophages are target cells of LPS 14]. After interaction with an endotoxic bacterium, the innate immunity is rapidly triggered, primarily via activation of toll-like receptor 4 (TLR-4)[15]. Activation of TLR-4 induces a multitude of proinflammatory cytokines via activation of transcription factors, nuclear factor κB (NF-κB)[16]. This prompt response provides a favorable environment for the synthesis and upregulation of both IL-1β and IL-6, which together contribute to the perpetuation of the inflammatory challenge.The pro-inflammatory response observed in septic shock is mediated by counter-regulatory molecules which attempt to restore the immunological equilibrium, amongst counter pro-inflammatory molecules include TNF, IL-1;alternatively, IL-10 an anti-inflammatory cytokine modulates the cascade and synthesis of inflammatory factors by lymphocytes T and macrophages[17].Also the rapid increase in TNF-α following LPS, which is already present after 15 minutes, promotes synthesis of other cytokines and the initiation of the acute-phase response, chemokine release and oxidative stress[18]. Interestingly, the production of the reactive oxygen and nitrogen species is an inherent property of activated immune cells. Inflammation can lead to oxidative stress and conversely. Thus, oxidative stress and inflammation are involved in a self-perpetuating cycle.It is known that a deficiency of dietary amino acids impair immune functions, increasing the susceptibility to an infectious disease. The role of aminoacids such as arginine and lysine is very important in the immune response; they regulate the activation of T and B lymphocytes, cellular redox status and production of cytokines[19] which can be cytotoxic substances in pro-inflammatory process. In this work we found that administration of NutriSim© 15 minutes prior or after LPS challenge diminishes significantly the level of serum cytokines. Thus supplementation with a nutritional substance containing specific amino acids possibly enhances immune system response. On the other hand, anti-inflammatory effect could be due to L-arginine, a semi-essential amino acid, which has been describe to play an important role in stress situations like sepsis[20]. Additionally, it has been demonstrate that arginine diminishes lipid peroxidation in patients with diabetes mellitus[21]. It has been demonstrated that patients with severe sepsis shown low levels of arginine amongst others like citrulline and ornithine[22]. This could suggest that availability of arginine supplementation could be useful as a probable potential therapeutic in cases on septic shock, though it hasn´t been demonstrated the relation between low levels of arginine and severity of sepsis.At this regard, it has been reported that natural antioxidants such as melatonin[23], quercetin[24], luteolin [25] and other antioxidants (butylated hydroxyanisole, tetrahydropapaveroline,nordihydroguiauretic acid, 10, 11-dihydroxyaporphine)[25] are potent inhibitors of the LPS-induced production of TNF-α production in vivo.Choline is a precursor of neurotransmitter acetylcholine and an essential nutrient; it has also been demonstrated in vitro that choline inhibit TNF release in macrophages, and this might suggest that choline may be have anti-inflammatory activity[26], although the efficiency of choline should be further investigated.NutriSim© administration in the absence of LPS does not altered the serum cytokines levels, which might suggest that this supplement does not exert a negative effect on the immune system.
5. Conclusions
- It has been already demonstrated that LPS increase the cytokines production in immune cells and it can modulate both pro- and anti-inflammatory response; in this work we demonstrate that the use of a nutritional supplement such as NutriSim©, can modulate an inflammatory response in experimental model of endotoxic shock. However, the mechanism of action by which this supplement exerts its beneficial effect is still unclear. Whether our findings may be of therapeutic value needs to be further investigated.
ACKNOWLEDGEMENTS
- The authors wish to thank the veterinary doctor Jose Blanco Fabela (Animal supply, CIBO-IMSS. Guadalajara, Jalisco, Mexico).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML