-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2012; 2(5): 67-73
doi: 10.5923/j.ajb.20120205.04
Effect of Melatonin on Lipid Barrier in Rats’ Skin
Natalya V. Kostiuk 1, Veronika V. Zhigulina 1, Maya B. Belyakova 1, Dzhulianna V. Leshchenko 1, Mikhail V. Miniaev 2, Ljudmila Y. Djachkova 1, Irina V. Namestnikova 1, Yuriy V. Abramov 3, Tatjana V. Volodina 3, Vladislav L. Kozeltsev 3
1Department of Chemistry and Biochemistry
2Research Center, Tver State Medical Academy, Tver, 170100, Russia
3The All-Russia Scientific Research Institute of the Medicinal and Aromatic Herbs (VILAR), Moscow, 117216, Russia
Correspondence to: Maya B. Belyakova , Department of Chemistry and Biochemistry.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work we studied the effect of intraperitoneal administration of melatonin in a dose of 1 mg per kg on lipids and microstructure of rats’ skin. A single injection of melatonin induced a change in lipid profile most notably in phospholipids and triacylglycerol fractions which contents varied oscillatory in opposite each other with a maximum divergence at 3 hours. By 24 hours there was a significant increase in triglycerides, and after 48 hours the lipid profile approached the initial values. After regular daily administration of melatonin for 6 days, the contents of almost all lipid fractions were significantly reduced (most in triacylglycerols and phospholipids) with growing level of free fatty acids. By 21st day the contents of lipids in the skin increased again, but did not reach the initial values. Reduction of lipid level was accompanied by prominent degenerative changes in the microstructure of the skin by the 6th day of experiment. In general, the administration of melatonin in any way caused the changes in skin’s lipid profile but after a single introduction parameters of lipid spectrum in two days were restored to their initial values, and a regular administration for 6 days led to sustained changes in lipid profile and structure of the skin.
Keywords: Melatonin, Lipids, Phospholipids, Skin
Article Outline
1. Introduction
- One of the main functions of the skin is to form a barrier between the organism and the environment. The skin is a protector from mechanical damage, UV radiation, chemicals, pathogens, loss of water and electrolytes[1]. Proper function of the skin barrier is mostly provided by lipids. These compounds are localized not only inside the cells, but also form complex organized structures that fill the intercellular space of the epidermis[1,2]. Age-dependent changes in the skin and some diseases are directly related to a malfunction of the skin barrier[3,4,5]. The skin's physiological condition and metabolism are regulated by endocrine and paracrine signaling. Skin cells contain receptors for neuropeptides, growth hormone, glucocorticoids, sex hormones, insulin-like growth factor, retinoids, eicosanoids, serotonin, melatonin and others[6]. Many of these compounds can be synthesized directly into skin cells. Over the past few decades pineal hormone melatonin is increasingly used in the treatment of circadian rhythms, cardiovascular therapy, cancer, and neurodegenerative disease[7,8,9]. It is one of the most evolutionarily conserved and pleiotropic hormone still active in humans and it has been implicated in vital skin functions such as hair growth, fur pigmentation as well as melanoma control[10]. Melatonin, as a multifunctional hormone, appears to regulate and modulate other functions in humans through the activation of its receptors and works as strong antioxidant that protects the DNA and prevents lipids peroxidation[11,12]. Currently, there are some proposals that melatonin as a highly lipophilic compound penetrates easily through cellular membranes and therefore is able to efficiently protect readily every intracellular structure including enzymes, proteins, lipids, mitochondria and the nucleus against oxidative damage[10,13].Most of investigations regarding the different aspects of melatonin confirm that it is highly efficient anti-aging factor with immunoenhancing properties reducing skin oxidant damage and cancerogenesis[12,14,15,16]. Melatonin was successfully used as a protective substance for the skin exposed to UVR, as well as compound protecting whole-body irradiated animals[17]. Recent reports showed that melatonin increases survival and decreases apoptosis of keratinocytes, fibroblasts and leukocytes subjected to UVR as a result of scavenging of free radicals, as well as inhibits apoptotic proteins and lipids peroxidation[18,19]. Melatonin is a scavenger of both oxygen- and nitrogen-based reactive molecules, including peroxynitrite anion and its decomposition products, including hydroxyl radical, nitrogen dioxide, and carbonate radical [20]. Besides its ability to direct scavenge radicals and radical products, melatonin also augments the activities of antioxidative enzymes, including glutathione peroxidase (GPx), superoxide dysmutase (SOD) and glutathione reductase[20,21].Enormous clinical interest was caused by the recentlypublished evidence which indicates the normalization of lipid parameters in blood by exogenous melatonin in patients with obesity, diabetes, and hypercholesterolemia[22,23,24,25]. As it turns out, melatonin affects the intensity of lipid metabolism in different cell types such as liver, fat, bone, and so on[26,27,28,29,30].Since the skin is also a typical target for the pineal hormones, we have assumed that melatonin along with other compounds may be involved in the maintaining of skin lipid barrier. The purpose of this study was to evaluate the lipid content in the skin of rats under acute and prolonged administration of melatonin.
2. Materials and Methods
2.1. Rat model
- The experiments were conducted on white male Wistar rats (200-250 g) in the summer period. The animals were kept on a standard diet under natural light exposure. All experimental procedures were conducted in accordance with the Rules of laboratory practice in the Russian Federation (2003) and with Principles on Good Laboratory Practice (OECD № ENV/MC/CHEM (98)17, 1997).Rats were decapitated under ether anesthesia. Skin samples (100-150 mg) of interscapular region cleared from hair and subcutaneous adipose tissue were used for preparation of lipid extracts, and their further qualitative and quantitative analysis.
2.2. Melatonin Administration
- Rats received intraperitoneal injections of melatonin in 1 ml of a sterile physiological solution with a dose of 1 mg/kg of body weight at 10 am. It is believed that in this dosage melatonin induces the most expressed changes of lipid and carbohydrate metabolism[31].Animals were decapitated 1, 2, 3, 4, 5, 6, 24 or 48 hours after injection of a single dose of melatonin. The control group consisted of intact animals which did not receive melatonin. In case of prolonged administration of melatonin, animals were decapitated after 6 or 21 days of daily regular injections of melatonin. In this case, apart from intact animals, groups of comparison received injections of physiological solution for 6 and 21 days.
2.3. Lipid Extraction
- Lipid extracts from the skin samples were obtained by Folch Method[32]. The extract was used for further qualitative and quantitative analysis of lipids.Fractionation of lipids was performed using the method of thin layer chromatography[33] on silica gel L 5/40 Chemapol. Separation of total lipids on the fractions was carried out in a solvent system of hexane - diethyl ether - methanol - acetic acid at a ratio of 9:2:0.2:0.3 by volume. Thereby the following classes of lipids were reliably determined: phospholipids (PL), diacylglycerols (DAG), cholesterol (C), free fatty acids (FFA), triacylglycerols (TAG), cholesteryl esters (CE). Separation of PL was carried out with chloroform – methanol - acetic acid - water in a ratio of 5:3:0.8:0.4 by volume. In this system, the following fractions of phospholipids were reliably identified: glycerol phosphate (GP), lysophospholipids (LPL), sphingomyelins (SPH), phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidic acid + cardiolipin (PA+CL). Detection was carried out by the exposure of dried chromatogram in iodine vapor. Elution of lipid fractions was performed by a mixture of chloroform and ethanol (3:2 by volume) for DAG, C, FFA, TAG, CE, and chloroform: ethanol:14N NН4ОН (5.6:4.2:0.2 by volume) for PL. Obtained extracts were dried and used for the quantitative determination of individual lipid fractions.
2.4. Quantitative Determination of Lipids
- Determination of total lipids (TL) and their fractions were carried out by heating of the dried extracts with concentrated H2SO4 for 15-20 minutes at 190-2000С[33]. After cooling down, the samples were diluted with water 1:1, and absorbance was measured at a wavelength of 490 nm (for total lipids) or 400 nm (for individual fractions). The contents of total lipids and individual lipid fractions in the samples were determined by means of calibration standards purchased from Sigma-Aldrich.Quantitative determination of total phospholipids (TPL) and their fractions was carried out on the content of lipid phosphorus using malachite green method in modification[34]. Dried extracts were mineralized with perchloric acid for 25 min at 225 °C. Mineralizates were dissolved in 1.5 ml of water, then 2 ml of dye reagent were added (1 volume of 4.2% solution of ammonium molybdate in 5 N HCl + 3 volumes of 0.2% solution of malachite green) and 0.1 ml of 1.5% solution of Tween-20. After 1 hour, absorbance was measured at wavelength of 670 nm. Phosphate was determined by previously plotted calibration graph.
2.5. Microscopy of Skin Samples
- For microscopic examination semi-thin sections (1 µm) were used. Samples were fixed in 10% formalin solution, washed in cacodylate buffer (pH 7,2-7,4), and postfixed by 1% solution of OsO4. After that the samples were dehydrated in ascending alcohol concentrations and embedded in epoxy resin Epon 812. Sections were stained with a three-dye (methylene blue, azure II, basic fuchsine), embedded in Canada balsam and examined under light microscope[35].
2.6. Statistical Analysis
- To demonstrate statistically significant differences between groups of control and experimental animals, Student's t-test was used with p-values less than 0.05.
3. Results
3.1. Single Administration of Melatonin
- After injection of melatonin, significant increase of total lipids in the skin was observed only after 24 hours (Table 1). The elevation was occurred mainly due to the contribution of TAG, whereas the contents of other lipid fractions were relatively constant. The subsequent reduction of TAG returned the quantity of total lipids to almost the initial level after 48 hours.Nevertheless, during the first 6 hours after administration of melatonin, the changes in the ratio of individual lipid fractions were not as distinct. In this period, the most significant oscillatory changes were observed in TAG and PL fractions, the amounts of which varied in opposite phase, accompanied with a slight increase in FFA (fig. 1) The character of changes in TL level was fully consistent with the changes in PL fraction. Thus, we can note that in the first hours after the administration of melatonin, most labile lipid components of skin are PL, and only after 24 hours the level of TL begins to be determined by change of TAG content.
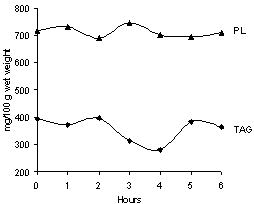 | Figure 1. Effect of single dose of melatonin on PL and TAG contents in skin |
3.2. Prolonged Administration of Melatonin
- At regular injections of melatonin, by the 6th day a significant decrease in the contents of almost all lipid fractions in the skin was coupled with growing levels of FFA (Table 3). However, in this case, the greatest decreases were also in the levels of TG and PL, against which the other fractions, except FFA, changed insignificantly. By the 21st days the contents of almost all lipid fractions were elevated again, but did not reach the initial values. Nevertheless, it can be explaned as a partial adaptation to the systematic introduction of the hormone. Among the phospholipids’ fractions the biggest changes were noted in the contents of PC, PE, PS (Table 4). In addition, lysophospholipids, the products of PL hydrolysis, were accumulated.
3.3. Microscopy of Skin
- After prolonged administration of melatonin the structure of the epidermis and dermis undergoes some changes. Figure 2 represents the microscopy image of the intact skin (A) and skin after 6 days of regular administration of melatonin (B). As the figure shows, the collagen bundles of the dermis under the influence of melatonin acquire a homogenous structure, their boundaries are poorly distinguished. Epidermis in some areas is damaged and flaky, while its fragments are visible on the surface of the dermis. Dyes stained the tissue poorly, which can be probably related to changes of its tinctorial properties.
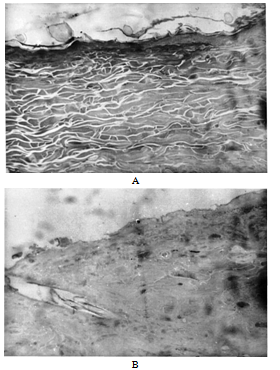 | Figure 2. The skin of intact rats (A) and after 6-day administration of melatonin (B), Magnification x200 |
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4. Discussion
- Our data suggest that melatonin may alter lipid metabolism in the skin of rats, and thus interferes with the functioning of the skin barrier. Effect of the drug depends on the duration of a course of injections. The response of the skin lipids to a single dose of melatonin is extended in time, but early alterations of parameters are detected even a few hours after injection, and full return to initial values does not occur even after two days. The dynamics of change is complex; oppositely directed changes in concentrations of major lipid fractions are observed. Prolonged uptake of melatonin results in a reduction of lipid level in the skin that may cause persistent disruption of its barrier function. This is indirectly confirmed by the results of microscopy of histological preparations of the skin.It is known that the lipid skin barrier is formed due to the functioning of the sebaceous glands and differentiating keratinocytes. Sebocytes and keratinocytes have lipoprotein lipase on their surface, LDL receptors, and various transporters of fatty acids[36,37]. Part of the skin lipids are of nutritional origin. However, skin cells are capable of independent synthesis and transformation of fatty acids. One can find a full set of enzymes necessary for the formation of triglycerides, phospholipids, sphingolipids, and cholesterol[36,37].Hormonal regulation of tissue lipid metabolism is carried out at different stages. Expected targets for endogenous and exogenous regulators are in a system of lipid transport in the blood, lipoprotein receptors, membrane transporters of lipids, quantity and activity of intra- and extracellular enzymes responsible for metabolism of these compounds[1,2,36]. It is not clear which of the potential targets can be used by melatonin to suppress production of lipids in the skin. However, data obtained by other researchers for different cell types, allow us to propose the following explanations.First, melatonin may impair the tissue supply of alimentary lipids by reducing the number of circulating lipoproteins, TAG, and C. Similar results were obtained in humans and various animals[6,38,39]. Second, melatonin is able to modify the transport of lipids through the plasma membrane. Thus, in mononuclear leukocytes of humans the hormone reduces the number of receptors for LDL, which leads to lower levels of intracellular cholesterol[40]. In adipocytes melatonin inhibits fatty acid transporters[41]. In postmenopausal women with normolipidemia prolonged administration of the hormone inhibits the activity of LP-lipase[8]. Finally, intracellular enzymes may serve as a target for melatonin. Thus, in adipocytes isolated from the inguinal fat pads, melatonin inhibits lipolysis induced by izoproterenol[42].Despite the progress made in deciphering the intracellular signaling of melatonin, the questions remain open regarding the mechanisms of lipid metabolism regulation by this hormone. Possibilities widely discussed in the literature include both direct action of melatonin[28,29] through specific receptors (membrane МТ1, МТ2 and nuclear RZR/RORα, RZR/ROR)[43] and effects mediated by other hormones (insulin, glucocorticoids, growth hormone, leptin, etc.)[41,44].Specialized melatonin receptors are present anywhere in the skin. Membrane and nuclear receptors have been found in fibroblasts, keratinocytes, melanocytes, the cells of hair follicles, eccrine glands, and the endothelium of blood vessels[27]. Thus, there is the potential of direct action of melatonin on lipid metabolism. Indirect evidence favouring this mechanism is reported in the present work as rapid changes in the skin content of TAG and PL in the first 6 hours after administration of melatonin.The possibility of indirect action of melatonin is indicated in the study by B. Bojková et al[45], where it has been found that prolonged administration of melatonin led to increased levels of corticosterone in the blood of male rats. Glucocorticoids negatively affect the skin barrier function, because they reduce lipid synthesis in the epidermis[46]. It has been shown[47] that melatonin, with the participation of receptors МТ1, suppresses insulin secretion by isolated pancreatic islets. Hypoinsulinemia also results in suppression of lipogenesis and stimulation of lipolysis.The action of melatonin, mediated by the endocrine glands and, thus, combined with the more or less long biosynthetic processes must be developed with a certain delay. This assumption is not excluded as a possible explanation for the significant growth of TAG level by 24 hours and its subsequent reduction to 48 hours after administration of melatonin reported here.
5. Conclusions
- Our study demonstrates a distinct effect of melatonin on the skin: the administration of melatonin, in any case, causes changes in the skin’s lipid profile but after a single injection the parameters of lipid spectrum are restored to their original values after two days, and a regular administration for 6 days leads to sustainable changes in lipid spectrum and the structure of the skin.Uptake of exogenous melatonin is one of the reasons for significant changes in tissue structure and, consequently, the barrier function of skin, causing in both a decrease total lipids of the skin, and a change its lipid profile. This fact may be considered as one of the side effects of melatonin preparations.
References
| [1] | Feingold, K. R. (2007) The role of epidermal lipids in cutaneous permeability barrier homeostasis. The Journal of Lipid Research, 48, 2531-2546. |
| [2] | Wertz, P. W. (2000) Lipids and barrier function of the skin. Acta Derm Venereol, 208, 7-11. |
| [3] | Pietrzak, A., Michalak-Stoma, A., Chodorowska, G. and Szepietowski, J. C. (2010) Lipid Disturbances in Psoriasis: An Update, Mediators of Inflammation, Article ID 535612:13. http://www.hindawi.com/journals/mi/2010/535612/ |
| [4] | Jungersted, J.M., Hellgren, L.I., Jemec, G.B. and Agner, T. (2008) Lipids and skin barrier function: a clinical perspective, Contact Dermatitis, 58(5), 255-262. |
| [5] | Elias, P. M., Williams, M. L., Holleran, W. M., Jiang, Y. J. and Schmuth, M. (2008) Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. The Journal of Lipid Research, 49, 697-714. |
| [6] | Zouboulis, C. C. (2004) The human skin as a hormone target and an endocrine gland. Hormones, 3(1), 9-26 |
| [7] | Zawilska, J. B., Skene, D. J. and Arendt, J. (2009) Physiology and pharmacology of melatonin in relation to biological rhythms. Pharm. Reports, 61, 383 – 410 |
| [8] | Yonei, Y., Hattori, A., Tsutsui, K., Okawa, M. and Ishizuka, B. (2010) Effects of Melatonin: Basics Studies and Clinical Applications. Anti-Aging Medicine, 7 (7), 85-91 |
| [9] | Kwon, K., Kim, J., Kim, M., Lee, J., Ignarro, L., Kim, H., Shin, C. and Han S. (2011) Melatonin synergistically increases resveratrol-induced heme oxygenase-1 expression through the inhibition of ubiquitin-dependent proteasome pathway: a possible role in neuroprotection. J Pineal Res, 50(2),110-23. |
| [10] | Kleszczyński, K., Hardkop, L. and Fischer T. Differential effects of melatonin as a broad range UV-damage preventive dermato-endocrine regulator (2011) Dermatoendocrinol, 3(1), 27–31. |
| [11] | Rosales-Corral, S., Lopez-Armas, G., Cruz-Ramos, J., Melnikov, V., Tan, D., Manchester, L., Munoz, R. and Reiter, R. (2012) Alterations in Lipid Levels of Mitochondrial Membranes Induced by Amyloid-β: A Protective Role of Melatonin. Int J Alzheimers Dis.2012, Article ID 459806. http://www.hindawi.com/journals/ijad/2012/459806/ref/ |
| [12] | Man'cheva, T., Demidov, D., Plotnikova, N., Kharitonova, T., Pashkevich, I. and Anisimov V. (2011) Melatonin and metformin inhibit skin carcinogenesis and lipid peroxidation induced by benz(a)pyrene in female mice. Bull Exp Biol Med, 151(3), 363-5. |
| [13] | Dauchy, R.T., Blask, D.E., Dauchy, E.M., Davidson, L.K., Tirrell, P.C., Greene, M.W., Tirrell, R.P., Hill, C.R., and Sauer, L.A. (2009) Antineoplastic effects of melatonin on a rare malignancy of mesenchymal origin: melatonin receptor-mediated inhibition of signal transduction, linoleic acid metabolism and growth in tissue-isolated human leiomyosarcoma xenografts. J Pineal Res, 47, 32-42. |
| [14] | Srinivasan, V., Pandi-Perumal, S., Brzezinski, A., Bhatnagar, K. and Cardinali, D. (2011) Melatonin, immune function and cancer. Recent Pat Endocr Metab Immune Drug Discov, 5(2),109-23. |
| [15] | Eşrefoğlu, M., Seyhan, M., Gül, M., Parlakpinar, H., Batçioğlu, K. and Uyumlu B. (2005) Potent therapeutic effect of melatonin on aging skin in pinealectomized rats. J Pineal Res, 39(3),231-7. |
| [16] | Tunali, T., Sener, G., Yarat, A. and Emekli, N. (2005) Melatonin reduces oxidative damage to skin and normalizes blood coagulation in a rat model of thermal injury. Life Sci, 6(11),1259-65. |
| [17] | Melatonin in the Promotion of Health (2011)[edited by] Watson, R.R./-2nd ed. – 564p. |
| [18] | Izykowska, I., Cegielski, M., Gebarowska E (2009) Effect of melatonin on human keratinocytes and fibroblasts subjected to UVA and UVB in vitro. In vivo, 23(5),739-45. |
| [19] | Fischer, T., Slominski, A., Zmijewski, M., Reiter, R. and Paus R. (2008) Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol, 17,713–730. |
| [20] | Korkmaz, A., Reiter, R., Topal, T., Manchester, L., Oter, S. and Tan, D. (2009) Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med, 15, 43-50. |
| [21] | Murawska-Ciałowicz, E., Jethon, Z., Magdalan, J., Januszewska, L., Podhorska-Okołow, M., Zawadzki, M., Sozanski T. and DzieRgiel, P. (2010) Effects of melatonin on lipid peroxidation and antioxidative enzyme activities in the liver, kidneys and brain of rats administered with benzo(a)pyrene, Experimental and Toxicologic Pathology 63, 1-2, 97. DOI : 10.1016/j.etp.2009.10.002 |
| [22] | Agil, A., Navarro-Alarcón, M., Ruiz, R., Abuhamadah, S., El-Mir, M.Y. and Vázquez, G.F. (2011) Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res, 50(2), 207-12. |
| [23] | Contreras-Alcantara, S., Baba, K. and Tosini, G. (2010) Removal of Melatonin Receptor Type 1 Induces Insulin Resistance in the Mouse. Obesity, 189, 1861–1863. |
| [24] | Nishida, S., Segawa, T. and Murai, I. (2002) Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of Delta-5 desaturase activity. J Pineal Res, 32, 26-33. |
| [25] | Hussain, S.A. (2007) Effect of melatonin on cholesterol absorption in rats. J.Pineal Res, 42, 267-271. |
| [26] | Nieminen, P., Käkelä, R., Mustonen, A.M., Hyvärinen, H. and Asikainen, J. (2001) Exogenous melatonin affects lipids and enzyme activities in mink (Mustela vison) liver. Comp Biochem Physiol C Toxicol Pharmacol, 128(2), 203-211. |
| [27] | Sanchez-Hidalgo, M., Lu, Z., Tan, D.-X., Maldonado, M. D., Reiter, R. J., and Gregerman, R.I. (2007) Melatonin inhibits fatty acid-induced triglyceride accumulation in ROS17/2.8 cells: implications for osteoblast differentiation and osteoporosis. American journal of physiology, 292 (6), 2208-2215 |
| [28] | Sauer, L.A., Dauchy, R.T. and Blask, D.E. (2001) Melatonin inhibits fatty acid transport in inguinal fat pads of hepatoma 7288CTC-bearing and normal Buffalo rats via receptor-mediated signal transduction. Life Sci, 68, 2835–2844. |
| [29] | Mullerwieland, D., Behnke, B., Koopmann, K. and Krone, W. (1994) Melatonin Inhibits LDL Receptor Activity and Cholesterol-Synthesis in Freshly Isolated Human Mononuclear Leukocytes. Biochemical and Biophysical Research Communications, 203 (1), 416-421. |
| [30] | Gonciarz, M., Gonciarz, Z., Bielanski, W., Mularczyk, A., Konturek, P., Brzozowski, T. and Konturek, S. (2012) The effects of long-term melatonin treatment on plasma liver enzymes levels and plasma concentrations of lipids and melatonin in patients with nonalcoholic steatohepatitis: a pilot study. J Physiol Pharmacol, 63(1), 35-40. |
| [31] | Van Cauter, E. (1998) Putative roles of melatonin in glucose regulation. Therapie, 53(5), 467-472. |
| [32] | Folch, J., Lees, M. and Stanley, G.H.S. (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem, 226, 497-509. |
| [33] | Kates, M. (1972) Techniques of Lipidology, In: Laboratory, Techniques in Biochemistry and Molecular Biology, Volume 3, Eds. WorK T.S., Work E. Amsterdam, North-Holland,. |
| [34] | Zhou, X. and Arthur, G. (1992) Improved procedures for the determination of lipid phosphorus by malachite green. J Lipid Res, 33(8), 1233-6. |
| [35] | Humphrey, C. D. and Pittman, F. E. (1974) A Simple Methylene Blue-Azure Ii-Basic Fuchsin Stain for Epoxy-Embedded Tissue Sections. Biotechnic &Histochemistry, 49(1), 9-14. |
| [36] | Smith, K. R. and Thiboutot, D. M. (2008) Sebaceous gland lipids: friend or foe? The Journal of Lipid Research, 49,271-281. |
| [37] | Feingold, K. R. (2009) The outer frontier: the importance of lipid metabolism in the skin. The Journal of Lipid Research, 50, 417-422. |
| [38] | Wang, S., Soni, K.G., Semache, M., Casavant, S., Fortier, M., Pan, L. and Mitchell, G.A. (2008) Lipolysis and the integrated physiology of lipid energy metabolism, Mol Genet Metab, 95(3), 17-26. |
| [39] | Wang H. and Eckel R.H. (2009) Lipoprotein lipase: from gene to obesity, Am J Physiol Endocrinol Metab, 297(2), 271-288. |
| [40] | Blasiole, D.A., Davis, R.A. and Attie, A.D. (2007) The physiological and molecular regulation of lipoprotein assembly and secretion. Mol Biosyst, 3(9), 608-19. |
| [41] | Wakatsuki, A., Okatani, Y., Ikenoue, N., Kaneda, C. and Fukaya, T. (2001) Effects of short-term melatonin administration on lipoprotein metabolism in normolipidemic postmenopausal women, Maturitas, 38(2), 171-177. |
| [42] | Kassayová, M., Marková, M., Bojková, B., Adámeková, E., Kubatka, P., Ahlersová, E. and Ahlers, I. (2006) The influence of long-term melatonin administration on basic physiological and metabolic variables of young Wistar:Han rats. Biologia, 61(3), 313-320. |
| [43] | Kostiuk, N.V., Zhigulina, V.V., Belyakova, M.B., Leschenko, D.V. and Minyaev M.V. (2011) Melatonin receptors and their agonists, Problems of biological, medical and pharmaceutical chemistry, 5, 49-58[In Russian]. |
| [44] | Zalatan, F., Krause, J. A. and Blask, D. E. (2001) Inhibition of Isoproterenol-Induced Lipolysis in Rat Inguinal Adipocytes in Vitro by Physiological Melatonin via a Receptor-Mediated Mechanism. Endocrinology, 142 (9), 3783-3790. |
| [45] | Bojková, B., Marková, М., Ahlersová, Е., Аhlers, I., Adámeková, Е., Kubatka, П. and Kassayová, М. (2006) Metabolic Effects of Prolonged Melatonin Administration and Short-Term Fasting in Laboratory Rats. Acta Vet.BRNO, 75, 21–32. |
| [46] | Slominski, A., Fischer, T. W., Zmijewski, M. A., Wortsman, J., Semak, I., Zbytek, B., Slominski, R. M. and Tobin, D. J. (2005) On the Role of Melatonin in Skin Physiology and Pathology. Endocrine, 27(2),137–148. |
| [47] | Arushanyan, E.B. and Arushanyan, L.G. (1991) Modulator properties of epiphyseal melatonin, Problems of endocrinology, 37(3), 65-68[In Russian]. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML