-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2012; 2(5): 62-66
doi: 10.5923/j.ajb.20120205.03
Haematological and Biochemical Effects of Metalaxyl Fungicide on Albino Mice
Wael M. Al-Amoudi
Biology Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia
Correspondence to: Wael M. Al-Amoudi , Biology Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Metalaxyl is a benzenoid fungicide used to control fungal diseases. The aim of this work is to investigate the effect of metalaxyl on haematological and biochemical parameters in male albino mice. In this study, animals were divided into two groups. The animals in the first group (20 mice) were given metalaxyl orally through a stomach tube at a dosage of 1/10 of the LD50 (which was equivalent to 130 mg/kg body weight) at a rate of three times per week for 4 continuous weeks. The second group was treated as a control in which the animals (20 mice) were given water orally. Treating mice with metalaxyl for 2 and 4 weeks induced a significant decrease in the RBC count (5.2±0.24), haemoglobin content (11.5±2.3) and the number of blood platelets (543±15.3). In contrast, metalaxyl treatment induced a significant increase in the WBC count (5.8±0.4). Serum triglyceride and cholesterol levels also increased while total protein and albumin levels decreased in the treated animals. Additionally, metalaxyl treatment resulted in a significant increase in the levels of transaminases, ALT and AST enzymes. The data also revealed a significant increase in serum creatinine in response to metalaxyl toxicity, which might be due to impaired kidney function resulting from fungicide exposure. It was concluded that these metalaxyl effects could be attributed to fungicide-induced oxidative stress, which in turn leads to signs of toxicity. This result was confirmed by other authors who obtained similar results by observing the effects of some fungicides. The premise of this study was that fungicides must be examined for their possible adverse effects on animals and humans before their application to agricultural fields.
Keywords: Metalaxyl, Mice, Haematological Parameters, Biochemistry, Transaminases
Article Outline
1. Introduction
- Pesticides are used extensively throughout the world to control agricultural pests and protect public health. Although these chemicals have many beneficial purposes, they can also cause adverse effects in both humans and animals. Pesticides have chronic health effects both as the sequelae of acute poisonings and through chronic exposure. Many studies have documented the adverse health effects of pesticide exposure on humans. There is also convincing evidence that pesticides play a role in human cancers. For example, epidemiological studies have linked insecticide exposure in the home to the development of brain cancer and leukaemia in children. There are several areas of concern that need to be extensively investigated. These chemicals are widely used in industry, agriculture, home and gardens for many different purposes, including the protection of seed grain during storage and germination[10]. Individuals who are exposed to these chemicals include agricultural workers, those living in proximity to farms/orchards or consumers who are exposed to pesticide residues in food[5]. Metalaxyl is a systemic benzenoid fungicide belonging to the most widely known member of the amide group. It is used in foliar spray mixtures for tropical and subtropical crops, as a soil treatment for the control of soil-borne pathogens, and as a seed treatment to control downy mildews, fungal diseases on fruits, cotton, soybeans, peanuts, ornamentals and grasses[10]. Metalaxyl is reported to have hazardous effects on mammalian animals[39].[15] reported that metalaxyl has cytogenetic effects on human and animal chromosomes only in vitro but not in vivo.[40] has also reported that feeding mice with metalaxyl over the long term induced hepatic injury.[25] has indicated that this chemical could lead to the development of cocarcinogenic potential in Swiss albino mice. Metalaxyl caused dose-dependent bradycardia, and at higher doses (250 and 300 mg/kg body weight), the sustained bradycardia led to cardiac arrest[23].[37] has reported that metalaxyl induced histological and biochemical alterations in the liver of albino mice. Moreover,[9] found that imidacloprid and metalaxyl induced in vitro micronucleus formation and sister chromatid exchange induction in human lymphocytes as well as in vivo micronucleus induction in the polychromatic erythrocytes of rat bone marrow, both separately or in combination with one another. Apoptosis and bax expression were observed in the hepatocytes of mice that were treated with metalaxyl[35]. In addition, residues of buprofezin, chlorpyriphos, metalaxyl, and myclobutanil were detected in incurred grape and wine samples[7]. The present study was undertaken to examine the effects of metalaxyl-induced stress on the haematological and biochemical activities of albino mice, as indicated by haemoglobin content, the number of blood platelets, and WBC count as well as serum triglyceride, cholesterol, total protein, albumin and creatinine levels. There were also analyses of the changes in transaminases, ALT and AST enzyme levels.
2. Materials and Methods
2.1. Animals and Treatments
- Male albino mice (Mus musculus) weighing 20±5 g were obtained from the animal house of King Abdel Aziz University, Jeddah, Saudi Arabia and then housed in plastic cages (40×30×16 cm) in the laboratory at a constant temperature (22±1°C) for at least one week before and during the experimental work period. They were maintained on a standard rodent diet, and water was available ad libitum. All experiments were performed in compliance with a guide for the care and use of laboratory animals (National Research Council, 1985). Animals were divided into 2 experimental groups.Group 1: Animals in this group (20 mice) were given metalaxyl orally using a stomach tube and a dose level of 1/10 LD50 (130 mg/kg body weight) three times per week for 4 continuous weeks. Group 2: Animals in this group were the controls. All animals (20 mice) were given water orally.
2.2. Biochemical Assays
- For the haematological study, blood was collected from both the control and treated animals after 2 and 4 weeks of treatment. The haematological parameters evaluated were the following: red blood cell count (RBC), haemoglobin value (Hb), white blood cell count (WBC) and blood platelet number, which were measured according to[8]. For the biochemical study, sera were obtained by centrifuging the blood samples and storing them at 20℃ until the assays could be completed. Total protein concentration was estimated by the method in[18], cholesterol levels were determined in the manner of[31], triglyceride levels were determined according to the technique of[12], and creatinine and albumin levels were determined using the methods in[14]. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were determined colourimetrically according to[29].
2.3. Statistical Analysis
- The results were expressed as the mean ± SD of each different group. The differences between the mean values were evaluated by ANOVA, which was followed by Student’s t test that was carried out using the Minitab 12 computer program (Minitab Inc., State College, PA).
3. Results and Discussion
- The data in table (1) show that there was a gradual decrease in erythrocyte count, haemoglobin content and the number of blood platelets in mice treated with metalaxyl. After 4 weeks of treatment, this decrease was statistically significant. In contrast, the leukocyte count was significantly increased after the fourth week of treatment with metalaxyl. These results are in agreement with those obtained from previous studies of the haematological effects of fungicides on mammalian animals.[13] reported that rats that were fed diets containing thiophanate-methyl fungicide at doses of 0, 12.8, 64, 320, 1600, or 8000 ppm for six months demonstrated a decreased erythrocyte count and lower haemoglobin content. Increased leukocyte counts were observed frequently at 6000 ppm. Repeated high doses of carbendazim fungicide were associated with liver toxicity and adverse effects on blood cells and in rats[22].[1] showed that male dogs that were fed capsules containing thiophanate-methyl fungicide showed decreases in their total erythrocyte counts as well as their haemoglobin and haematocrit values at the highest dose.[4] noted that a blood analysis of rabbits treated with lambda-cyhalothrin revealed a significant decrease in red blood cell and white blood cell counts, haemoglobin concentration and lymphocytes, while mean corpuscular haemoglobin concentration, mean corpuscular volume, neutrophils, monocytes and eosinophils all increased. Collectively, the decrease in the erythrocyte count and haemoglobin content recorded in the present work indicated that metalaxyl-treated mice were anaemic. According to[19], this anaemia is due to (a) increased blood as a result of the accelerated red cell destruction by haemolytic agents or rapid cell removal from an abnormality of cell shape or over-activity of the spleen (b) a quantitative decrease in blood formation as a result of a quantitative decrease in red marrow from aplasia or a quantitative decrease in marrow activity from a deficiency in the substances necessary for normal bone marrow activity.
|
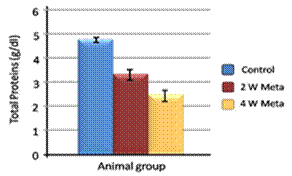 | Figure 1. Effect of metalaxyl on total proteins |
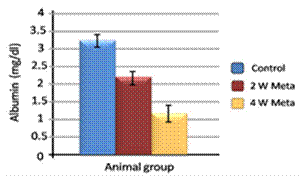 | Figure 2. Effect of metalaxyl on total albumin |
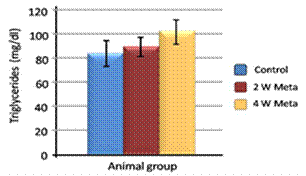 | Figure 3. Effect of metalaxyl on triglycerides |
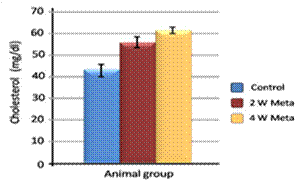 | Figure 4. Effect of metalaxyl on cholesterol |
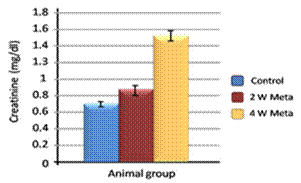 | Figure 5. Effect of metalaxyl on creatinine |
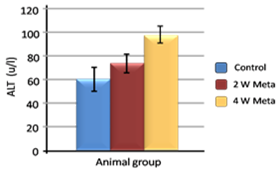 | Figure 6. Effect of metalaxyl on ALT |
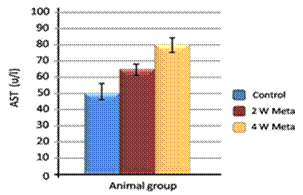 | Figure 7. Effect of metalaxyl on AST |
4. Conclusions
- Metalaxyl exposure leads to abnormal haematological and biochemical activities as well as induced oxidative stress in mice, which in turn leads to observable toxicity. Treating mice with metalaxyl for 2 and 4 weeks induced a significant decrease in RBC counts, haemoglobin content and the number of blood platelets. In contrast, the effects of metalaxyl led to a significant increase in WBC counts. Serum triglyceride and cholesterol levels were also increased while total proteins and albumin were decreased in the treated animal. Additionally, metalaxyl exposure led to a significant increase in the production of transaminases, ALT and AST enzymes.The results also revealed a significant increase in serum creatinine levels in response to metalaxyl toxicity, which might be due to kidney function impairment by the fungicide that was used for this study. It is concluded that these metalaxyl effects may be attributed to induced oxidative stress, which in turn led to the toxicity that was recorded in this article. This interpretation was confirmed by other authors who obtained similar results from studying the effects of fungicides within the same class. The present study suggests that fungicides must be examined for their possible adverse effects on animals and humans before their application to agricultural fields.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML