-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2012; 2(5): 51-55
doi: 10.5923/j.ajb.20120205.01
Hypothesis of Early Development of Chicken Embryos
T. O. Azarnova , I. S. Yartseva , A. E. Bobilkova
Moscow State Academy of Veterinary Medicine, Biotechnology Named by K.I. Skryabin
Correspondence to: I. S. Yartseva , Moscow State Academy of Veterinary Medicine, Biotechnology Named by K.I. Skryabin.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work we set the following goal: to study the influence of a complex usage of such natural metabolites as Kolamin, succinic acid and Ribav upon the acceleration of chicken embrio development, as well as their hatching and viability indices. For the estimation of biochemical indices, standard methods were used, according to the data in the scientific literature (I.P.Kondrakhin, 1985; M.I.Prokhorova, 1982; V.V.Menshikov, 1987; B.I.Antonov, 1991). As a result we found that aerosol processing of incubational eggs by the complex of natural metabolites: ethanolamine, succinate and Ribav makes it possible to neutralize free radical reactions and lipid peroxidation and, as a result, the hypoenergetic conditions in the organism of the fowl, which produces an opportunity to correct the metabolic processes in the critical periods of fetal development. All this causes the optimization of internal homeostasis, and therefore mitigates the negative processes in these stages. In connection with that we can make a conclusion about an early development of chicken embrios, as well as about a positive influence of the metabolites under analysis upon the incubation biocontrol markers.
Keywords: Chickens, Oxidative Stress, Lipid Peroxidation, Kolamin, Succinic Acid, Ribav, The Critical Periods of Incubation
1. Introduction
- In the process of embryogenesis the aniage and formation of all organs, tissues and systems of the fowl take place. That is why this period is the most important in the life of a subject.As is known, during some periods of incubation the mortality of embryos is especially high, which is determined by abrupt saltatory alterations in the organism (for example, the switch from allantoic to lung respiration) that result in high tension and often even failure of many metabolic processes[6]. All this leads to excessive energetic expenses. Hypoenergetic statuses in these periods are to a great extent determined by a “breakdown” of the mitochondrial respiratory chain, due to which excessive production of free radicals and active oxygen forms takes place[2]. All this predetermines not only a high mortality rate but at least a deceleration of embryo development in the strongest organisms. The main periods in which these negative phenomena take place are called critical – they usually occur on the 4-5th, 14-15th and 19-20th days of incubation[12]. Besides, when speaking about the reasons for mortality rise during these critical periods, many researchers associate them not only with the natural physiological reasons. They also link the first period to a long-time storage of the eggs prior to incubation or to their overheating at the beginning of it, the second one – to the low quality of incubational eggs (due to lack of vitamins and other nutrients) and the third one – to various disruptions of incubation conditions[14]. It is also pointed out that at the end of incubation the mortality rate is usually three times as high as at the beginning. The embryos dying in the critical periods are ascribed to the categories of incubational waste: the blood ring, the dead-in-shell and the addled eggs[1]. However, even if the chick outstays these critical periods, they undoubtedly have a negative influence upon the embryo’s development and formation, which, as was pointed out earlier, is directly connected with theincrease of the incubation time, various pathologies in the development of organs, poor exterior characteristics of the fowl and a high mortality rate, especially during the first weeks of life[5].
2. Objectives
- Thus, the aim of this work is to reveal the possibility of using the complex of metabolites: monoethanol amine, succinic acid and Ribav for the neutralization of free radical processes, lipid peroxidation and hypoenergetic statuses, with the main goal of correcting the critical periods of embryo development.Free radicals are produced in the organism even without any pathologies, having their own important purposes in the organism[7]. But together with a disruption of the general homeostasis and the influence of negative environmental factors, as well as the loss of nutrients and microelements, the elaboration of free radicals by the organism becomes many times higher, which causes the development of pathological processes in the organism of the embryo, later on having an effect on the viability and productivity of the adult organisms. Thus, one of the most valuable consequences of the excessive free radical synthesis is lipid peroxidation that leads to the cell membrane destabilization and the dysfunction of the cell[9].
3. Methods
- To prevent the abovementioned conditions, we used the main component of the membrane phospholipids –monoethanol amine, which, through the transformation into choline, can participate in the functioning of the mitochondrial respiratory chain[4]. It is important to point out that in order to make such transformations effective this biologicallyactive substance (BAS) needs the assistance of an energetic substrate[10]. Taking into account the experience of the previous researchers, a universal natural metabolite that is involved into the tricarboxylic acid cycle and the biological oxygenation was selected for this purpose – that is succinic acid, which, if necessary, can also be used by the organism in the synthesis of various substances[8]. Considering also the fact that when the peroxidation process is highly intensive there appear lipofuscins (Shiff’s bases) made from malondialdehyde and other aldehydes with protein structures, which happens due to the dysfunction of the latter, it was decided to insert a multicomponent preparation Ribav that mostly consists of essential and nonessential amino acids. It is important to remember that for a growing organism that is subject to stress any monomers of protein are essential[11].
4. Results
- In a series of experiments we proved the possibility of using the complex of biological stimulators and elaborated the application scheme for kolamin, succinic acid and Ribav by means of one-time aerosol processing of eggs before setting them for incubation.For the research an experimental group and a control one were selected (306 eggs in each) by the principle of analogy taking into account the laying time, the weight, the age of the parental stock and the storage term.
|
5. Discussion
- Thus we can suppose that the level and intensity of the metabolic processes and the tendencies traced in the experimental group are optimal, considering the fact that the experimental chickens are better developed and their viability is higher during a long period of life, whereas their embryonic development is shorter, at the same time being well-balanced.All the abovementioned proves that the suggested mixture has a marked antioxidant and metabolism-stimulating property. Evidently this is what the embryo needs for a balanced development without any delay. This is confirmed by the data from Table 3.
|
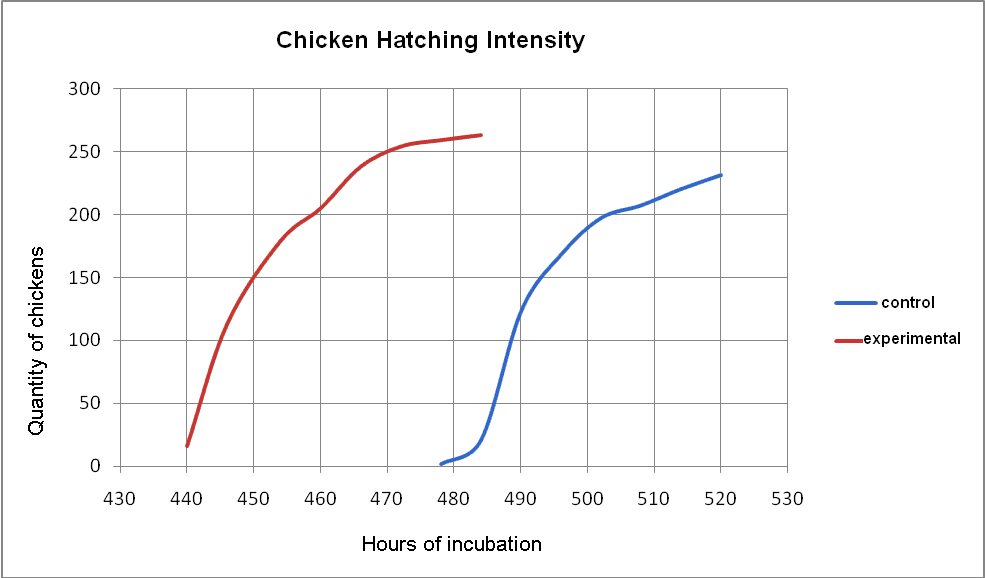 | Figure 1. Hatching Intensity of Chickens |
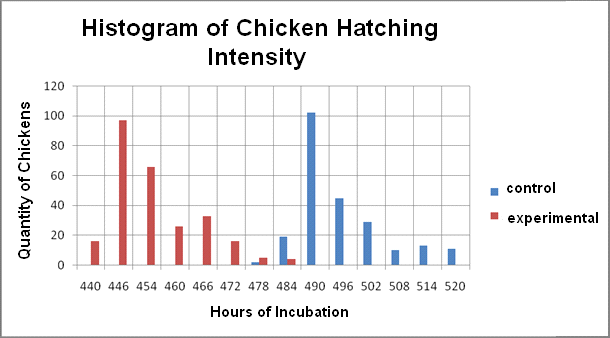 | Figure 2. Histogram of Chicken Hatching Intensity |
6. Conclusions
- Based upon all this, it is possible to suggest a hypothesis of earlier development of the embryos, due to the fact that, as our research shows, the complex of metabolites under analysis makes the process of embryogenesis 1.5 days faster. The embryogenesis of chickens (and probably of other living organisms) lasts longer than it is naturally determined. This happens due to simultaneous influence of various stress factors upon the organism of the embryo that cause destabilization of the homeostasis. Besides, there are critical periods in the process of embryogenesis in which the mortality rate is especially high. All this is a result of disruptions in many metabolic processes and of changes in their intensity. The latter lead to excessive energetic expences (in order to keep the homeostasis at the optimal level) as well as to full or partial loss of ATP in several processes[15]. The hypoenergetic statuses in these periods are to a great extent caused by the dysfunction of the mitochondrial respiratory chain, which leads to an excessive production of free radicals and active oxygen forms. This disturbs the biological oxygenation function and the metabolism as a whole, leading to hypoxia, deactivation of enzymes, destruction of receptors and hormones. All this undoubtedly slows down the development of stronger embryos and kills weaker ones[6]. The neutralization of free radical reactions and lipid peroxidation (and, as a result, the hypoenergetic statuses) makes it possible to correct the metabolic processes in the organism of the fowl not only during the main critical periods in the embryogenesis, but also in any stress situations, which keeps the inner homeostasis at the optimal level and, consequently, mitigates the negative processes. As a result, the organism of the chicken does not have to enlist all its efforts and to spend nutritional components on additional synthesis of ATP of which it did not receive enough due to disturbances in the functioning if the mitochondrial respiratory chain. All this enables the organism to shorten the terms of its embryogenesis in a natural way. It is worth mentioning that the metabolites under analysis can also be used for other bird species (the quail, the pelican and fancy hen breeds), as well as for the processing of plant seeds. Our latest research shows a high effectiveness of the metabolites for these biological objects. Taking this into consideration, we can conclude that the preparations under analysis can be widely used, due to the fact that the processes of biological oxygenation are identical both for the plants and the animals. Further on it can be possible to apply the worked out technologies for industrial poultry farming because it is economically justified.
References
| [1] | Yermolova, Yu.S. Processing of Chicken Eggs by Biologically Active Substances for the Stimulation of Chickens’ Resistance at Various Stages of Ontogenesis: Dissertation Abstract of Candidate of Biological Sciences// Moscow. – 2003. – p.23 |
| [2] | Zhuravlev, A.I. Antioxidants. Free Radical Pathology/ A.I.Zhuravlev, S.M.Zubkova// Moscow. – Moscow State Academy of Veterinary Medicine and Biotechnology named by K.I.Skryabin. – 2008. – 272 pp. |
| [3] | Isyangulova, R.Kh. The Substance and Energy Metabolism and Meet Productivity of Bulls in case of Feeding them with Mitugen, Kolamin and their Complexes in Stress Periods: Dissertation Abstract of Candidate of Agricultural Sciences// Orenburg. – 2008. – p.20 |
| [4] | Kinyabulatova, R.Kh. The Usage of Feed Preparation Mival and Kolamin for the Correction of Stress Adaptation and the Increase in Raising Effectiveness of Meet-type Bulls: Dissertation Abstract of Candidate of Agricultural Sciences// Orenburg. – 2009. – p.25 |
| [5] | Krasnobaev, Yu.V. Processing of Eggs of Meet-type Hens by the Ecologically Safe Preparation Helavit for the Stimulation of Embryonal and Postembryonal Development of Broyler Chickens: Dissertation of Candidate of Biological Sciences// Moscow. – Moscow State Academy of Veterinary Medicine and Biotechnology named by K.I.Skryabin. – 2009. – p.147 |
| [6] | Karput, I.M., Babina, M.P. Inner Noncontagious Diseases of Fowl// Minsk/ Data Processing Computer Center “Minfina”. – 2011. – 176 pp. |
| [7] | Kukhta, V.K., Morozkina, T.S., Oletskiy, E.I. Biological Chemistry// Moscow/ BINOM. – 2008. – 687 pp. |
| [8] | Marry, R., Grenner, D., Meyes, P. Human Biochemistry// Moscow/ Mir. – 2009. – 129 pp. |
| [9] | Marshall, V.J, Bangert S.K. Clinical Biochemistry. – 6th ed., revised and updated. – Moscow/BINOM. – 2011. – 408 pp. |
| [10] | Ottaway, P.B. Enrichment of Food Products and Biologically Active Substances: Technology, Safety and Regulatory System// St. Petersburg/ Professia. – 2010. – 312 pp. |
| [11] | Severin, E.S. Biochemistry// Moscow/ GEOTAR-MEDIA. – 2010. – 384 pp. |
| [12] | Soldatova I.B. Development and Metabolism of Chicken Embryos in case of Sound Stimulation// Ontogenesis. 2011. Vol.42, 4. |
| [13] | Tkachuk, V.A. Clinical Biochemistry. – 3rd ed., revised and updated. – Moscow/ GEOTAR-MEDIA. – 2008. – 454 pp. |
| [14] | Fisinin, V.I. Effective Defense against Stress in Poultry Farming: from Vitamins to Vitagens/ Fisinin, V.I., Surray, Peter// Fowl and Poultry Products// Moscow. – 2011, 5. – pp.23-26. |
| [15] | Yarovan, N.I. Biochemical Aspects of Evaluation, Diagnostics and Preventive Measures of Technological Stress in Agricultural Animals: Dissertation Abstract of Doctor of Biological Sciences// Moscow – 2008. – pp.3-39. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML