| [1] | Steverding, D., and Tyler, K.M., 2005, Novel antitrypanosomal agent. Expert Opinion on Investigational Drug., 14(8), 939-955 |
| [2] | Perez-Morga, D., 2007, Human resistance to African trypanosoma infections. Bulletin et mémoires de l'Académie royale de médecine de Belgique, 162(7-9), 381-386 |
| [3] | Hoet, S., Opperdoes, F.R., Brun, R., Quetin-Leclercq, J., 2004, Natural products active against African trypanosomes: a step towards new drugs. Nat. Prod. Rep., 21(3), 353-364 |
| [4] | Adewumi, C.O., Agbedahunsi, J.M., Adebajo, A.C., Aladesanmi, A.J., Murphy, N., and Wando, J., 2001, Ethno-veterinary medicine: screening of Nigerian medicinal plants for trypanocidal properties. J. Ethnopharmacol., 77, 19-24 |
| [5] | Atawodi, S.E., Bulus, T., Ibrahim, S., Ameh, D.A., Nok, A.J., Mamman, M., and Galadima, M., 2003, In vitro trypanocidal effect of methanolic extract of some Nigerian savannah plants. Afri. J. Biotech., 2(9), 317-321 |
| [6] | Bizimana, N., Tietjen, U., Zessin, K.-H., Diallo, D., Djibril, C., Melzig, M.F., and Clausen, P.–H., 2006, Evaluation of medicinal plants from Mali for their in vitro and in vivo trypanocidal activity. J. Ethnopharmacol., 103(3), 350-356 |
| [7] | Freiburghaus, F., Jonker, S.A., Nkuna, M.H.N., Mwasunbi, L.B., and Brun, R., 1997, in vitro trypanocidal activity of some rare Tanzanian medicinal plants. Acta Trop., 67, 181- 185 |
| [8] | Freiburghaus, F., Kaminsky, R., Nkunya, M.H. and Brun, R. (1997). Evaluation of African medicinal plants for their in vitro trypanocidal activity. J. Ethnopharmacol., 55(1), 1-11 |
| [9] | Freiburghaus, F., Ogwal, E.N., Nkunya, M.H., Kaminsky, R., and R Brun, R., 1997, In vitro antitrypanosomal activity of African plants used in traditional medicine in Uganda to treat sleeping sickness. Trop. Med. & Internat. Health., 1(6), 765-771 |
| [10] | Hoet, S., Opperdoes, F.R., Brun, R., Adjakidjé, V., and Quetin-Leclercq, J., 2004, In vitro antitrypanosomal activity of ethnopharmacologically selected Beninese plants. J. Ethnopharmacol., 91, 37–42 |
| [11] | Youan, B.B.C., Coulibaly, S., Miezan, T.B., Doua, F., and Bamba, M., 1997, In vivo evaluation of sixteen plant extracts on mice inoculated with Trypanosoma brucei Gambiense. Bull. World Health Org., 75, 343-348 |
| [12] | H.M. Burkill, The Useful Plants of West Tropical Africa. 2nd Ed., Volume 1, Families A– D. Royal Botanic Gardens, Kew, Richmond, United Kingdom., 1985 |
| [13] | Abubakar, A., Iliyasu, B., Yusuf, A.B., Onyekwelu, N.A., Igweh, A.C., Shamaki, B.U., Afolayan, D.O., and Ogbadoyi, E.O., 2005, Antitrypanosomal and hematological effects of selected Nigerian medicinal plants in Wistar rats. Biokemistri., 17, 95-99 |
| [14] | Atawodi, S.E., Ameh, D.A., Ibrahim, S., Andrew, J.N., Nzelibe, H.C., Onyike, E., Anigo, K.M., Abu, E.A., James, D.B., Njoku, G.C., Sallau, A.B., 2002, Indigenous knowledge system for treatment of trypanosomiasis in Kaduna state of Nigeria. J. Ethnopharmacol., 79, 279-282 |
| [15] | Atawodi, S.E., and Alafiatayo A.A., 2007, Assessment of the phytochemical and antitrypanosomal properties of some extracts of leaves, stem and root bark of Landolphia sp., P. Beauv. J. Ethnopharmacol., 114(2), 207-211 |
| [16] | Asuzu, I.U., and Chineme, C.N., 1990, Effects of Morinda lucida leaf extracts on Trypanosoma brucei brucei infection in mice. J. Ethnopharmacol., 30, 307-313 |
| [17] | Asuzu, I.U., and Ugwuja, M.O., 1989, A preliminary study of the biological activities of the bark extract of Piliostigma thonningii (Schum) in mice. Phytother. Res., 35, 209-211 |
| [18] | Ibrahim, M.A., Njoku, G.C., and Sallau, A.B., 2008, In vivo activity of stem bark aqueous extract of Khaya senegalensis against Trypanosoma brucei. Afri. J. Biotech., 7(5), 661-663 |
| [19] | Igweh, A.C., and Onabanjo, A.O., 1989, Chemotherapautic effects of Annona senegalensis in Trypanosoma brucei brucei. Ann. Trop. Med. Parasitol., 83, 527-534 |
| [20] | Maikai, V.A., Nok, J.A., Adaudi, A.O., and Alawa, C.B. 2008, In vitro antitrypanosomal activity of aqueous and methanolic crude extracts of stem bark of Ximenia americana on Trypanosoma congolense. J. Med. Plants Res., 2(3), 055-058 |
| [21] | Nok, A.J., Ibrahim, S., Arowosafe, S., Longdet, I., Ambrose, A., Onyenekwe, P.C., and Whong, C.Z., 1995, The trypanocidal effect of Cannabis sativa constituents in experimental animal trypanosomiasis. Vet. & Human Toxicol., 36(6), 522-524 |
| [22] | Nok, A.J., Williams, S., and Onyenekwe, P.C., 1996, Allium sativum-induced death of African trypanosomes. Parasitol. Res., 82(7), 634-637 |
| [23] | Nok, A.J., Esievo, K.A.N., Lingdet, I., Arowosafe, S., Onyenekwe, P.C., Gimba, C.E., and Kagbu, J.A., 1993, In vitro activity of leaf extracts against Trypanosoma brucei brucei. J. Clin. Biochem. Nutr., 15, 113-118 |
| [24] | Nwodo, N.J., Brun, R., and Osadebe, P.O., 2007, In vitro and in vivo evaluation of the antitrypanosomal activity of fractions of Holarrhena africana. J. Ethnopharmacol., 113(3), 556-559 |
| [25] | Nwosu, C.O., and Agbede, R.I.S., 2009, Antitrypanosomal effects of aqueous extract of Ocimum gratissimum (Lamiaceae) leaf in rats infected with Trypanosoma brucei brucei. Afri. J. Trad. Compl. & Alt. Med., : AJTCAM 6(3), 262-267 |
| [26] | Ogbadoyi, E.O., Akinsunbo, A.O., Adama, T.Z., and Okogun, J.I., 2007, In vivo Trypanocidal activity of Annona senegalensis leaf extract against Trypanosoma brucei brucei. J. Ethnopharmacol., 112, 85-89 |
| [27] | Ogbunugafor, H.A., Okochi, V.I., Okpuzor, J., Adedayo, T., and Esue, S., 2007, Mitragyna ciliata and its trypanocidal activity. Afri. J. Biotech., 6(20), 2310-2313 |
| [28] | Onyeyili, R.A., and Egwu, G.O., 1995, Chemotherapy of Africa trypanomiasis: A historical review. Protozool., 5, 229-243 |
| [29] | Owolabi, O.A., Makanga, B., Thomas, E.W., Molyneux, D.H., and Oliver, R.W., 1990, Trypanocidal potentials of Africa woody plants in vitro trials of Khaya grandifoliolium seed extracts. J. Ethnopharmacol., 30, 227-231 |
| [30] | Wosu, L.O., and Ibe, C.C., 1989, Use of extracts of Picrilima nitida in the treatment of experimental trypanosomiasis : A preliminary study. J. Ethnopharmacol., 25, 263-268 |
| [31] | Wurochekke, A.U., and Nok, A.J., 2004, In vitro antitrypanosomal activity of some medicinal plants used in the treatment of trypanosomiasis in Northern Nigeria. Afri. J. Biotech., 3(9), 481-483 |
| [32] | Shuaibu, M.N., Wuyep, P.T.A., Yanagi, T., Hirayama, K., Ichinose, A., Tanaka, T., and Kouno, I., 2008, Trypanocidal activity of extracts and compounds from the stem bark of Anogeissus leiocarpus and Terminalia avicennioides. Parasitol. Res., 102 (4), 697-703 |
| [33] | A. Mann, M. Gbate, and A. Nda-Umar, Medicinal and Economic Plants of Nupeland, Jube-Evans Books and Publications, Bida, Niger State, Nigeria, 2004 |
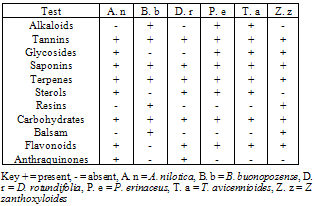
| [34] | Mann, A., Egwim, E.C., Banji, B., Umar, A.N., Gbate, M., and Ekanem, J.T., 2009, Efficacy of Dissotis rotundifolia on Trypanosoma brucei brucei infection in rats. Afri. J. Biochem. Res., 3(1), 005-008 |
| [35] | J.B. Harborne, Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. London, Chapman and Hall Ltd, 1989 |
| [36] | A. Sofowora, Medicinal Plants and Traditional Medicine in Africa, Ibadan, Spectrum Books, 1993 |
| [37] | G.E. Trease, and W.C. Evans, Pharmacognosy, 13th Ed. London, Bailliere Tindall, 1989 |
| [38] | Wright, C.W., and Phillipson, J.D., 1990, Natural products and the development of selective antiprotozoal drugs. Phytother. Res., 4, 127-139 |
| [39] | Hoet, S., Pieters, L., Muccioli, G.G., Habib-Jiwan, J.-L., Opperdoes, F.R., and Quetin-Leclercq, J., 2007, Antitrypanosomal Activity of Triterpenoids and Sterols from the Leaves of Strychnos spinosa and Related Compounds. J. Nat. Prod., 70(8), 1360 -1363 |
| [40] | Hoet, S., Stévigny, C., Block, S., Opperdoes, F.R., Colson, P., Baldeyrou, B., Lansiaux, A., Bailly, C., Quetin-Leclercq, J., 2004, Alkaloids from Cassytha filiformis and Related Aporphines: Antitrypanosomal Activity, Cytotoxicity, and Interaction with DNA and Topoisomerases. Planta Med., 70 (5), 407-513 |
| [41] | Merschjohann, K., Sporer, F., Steverding, D., and Wink, M., 2001, In vitro Effect of Alkaloids on Bloodstream forms of Trypanosoma brucei and T. congolense. Planta Med., 67, 623-627 |
| [42] | Sülsen, V.P., Cazorla, S.I., Frank, F.M., Redko, F.C., Anesini, C.A., Coussio, J.D., Malchiodi, E.L., Martino, V.S., and Muschietti, L.V., 2007, Trypanocidal and Leishmanicidal Activities of Flavonoids from Argentine Medicinal Plants. Am. J. Trop. Med. Hyg., 77(4), 654–659 |
| [43] | Nok, A.J., 2002, Azaanthraquinone inhibits respiration and in vitro growth of long slender bloodstream forms of T. congolense. Cell Biochem. and Function., 20, 205-212 |
| [44] | R. Carver, Chemotherapy of Helminthiasis. Pergamon Press 1973, vol .1 |
| [45] | Legros, D., Evans, S., Maino, F., Enyarel, J.C.K., and Mbulanuberi, D., 1999, Risk factors for treatment failure after melarsoprol for Trypamosoma brucei gambianse Trypanosomiasis in Uganda. J. Trans. Royal Soc. Trop. Med. & Hyg., 93 (4), 439 – 442 |
| [46] | Tijani, A.Y., Uguru, M.O., Salawu, O.A., Abubakar, A., Onyekwelu, N.O., and Akingbasote, J.A., 2009, Effect of Faidherbia albida on some biochemical parameters of rats infected with Trypanosoma brucei bruce. Afri. J. Pharm. Pharmacol. 3(1), 026-030 |
| [47] | Ogbadoyi, E.O., Ukoha, A.I., and Keywalabe, E., 1999, Anemia in experimental African Trypanosomiasis., The J. Protozool. Res., 9(2), 55-63 |
| [48] | Mamo, E., and Holmes, P., 1975, the erythrokinetics of zebu cattle chronically infected with T. congolense. Res. in Vet. Sci., 18, 105-106 |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML