-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Aerospace Sciences
p-ISSN: 2169-8872 e-ISSN: 2169-8899
2016; 4(1): 9-13
doi:10.5923/j.aerospace.20160401.02

Performance of Epoxy and Nanodiamond Exfoliated Montmorillonite Nanocomposite
Ayesha Kausar
Nanoscience and Technology Department, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanoscience and Technology Department, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This report investigates the possibility of improving the mechanical properties of triglycidyl para-amino phenol (TGAP) epoxy through dispersion of nanodiamond (ND) and montmorillonite-nanodiamond (MMT-ND) filler. The montmorillonite was first intercalated with octadecylamine followed by nanodiamond intercalation to form MMT-ND. Both the pristine nanodiamond as well as MMT-ND was employed as filler to form nanocomposite with triglycidyl para-amino phenol. In this case, diethyltoluene diamine was used as hardener. It was found that the presence of nanofiller steadily decreased the β-transition temperature of TGAP/ND and TGAP/MMT-ND systems. Atomic force micrographs have shown fine particle dispersion in nanocomposite. The effect of filler concentration on mechanical properties of epoxy resins was also analyzed. TGAP/ND 1-5 system showed increase in modulus from 1676 to 3074 MPa, while greater modulus increase was seen for TGAP/MMT-ND 1-5 system in the range of 2388-3408 MPa. The balanced properties justified the application of novel epoxy nanocomposites in aviation industry.
Keywords: Epoxy, Montmorillonite, Nanodiamond, Intercalation, Modulus
Cite this paper: Ayesha Kausar, Performance of Epoxy and Nanodiamond Exfoliated Montmorillonite Nanocomposite, International Journal of Aerospace Sciences, Vol. 4 No. 1, 2016, pp. 9-13. doi: 10.5923/j.aerospace.20160401.02.
Article Outline
1. Introduction
- In aircraft and propulsion system, the main attention of airplane designers was on the achievement of lightweight materials [1]. Because of larger part to limited ability of propulsion systems, absolute minimum weight was essential for practical flight from 1903 to 1930. Subsequently, the basic need for material choice in both aircraft and engines is strength to weight ratio. While the concern remains to be first order of significance, light weight is not only sufficient but necessary. Recent criteria for design is very complex and thus new design routes as well as increased constituents and processing routes are required by winning products [2]. A well-recognized family of thermosetting polymers is epoxy resins. They have been extensively employed in numerous industrial fields mainly high performance adhesives, coatings and other engineering applications due to their remarkable features. Moreover, the cured epoxy resin possesses low impact strength, low fracture toughness, poor resistance to crack initiation, and growth. Over the past decade numerous investigations have been carried out to find route for epoxy matrix toughening especially by the addition of second phase mainly elastomeric or rigid constituents [3-5]. Currently, it was found that the nanoclay fillers have high modulus (170 GPa) and high aspect ratio (200-1000). Owing to their excellent properties they have developed a new route for enhancement of mechanical potential such as impact resistance and toughness of cured epoxy matrix [6]. Due to their low cost and ease of availability, clays have fascinated remarkable interest. Additionally, the probability of achievement of properties with nanoclay was achieved with microscopic fillers. For aerospace applications and automotive industries (in which composite material density is required), low filler content is particularly needed [7]. Therefore, the low filler content is the demand of perfect structure of polymer layered silicate nanocomposites. Thus, clay act as conventional filler than that of exfoliated nanocomposite. In order to restrict the cracks propagation and increase the surface contact between clay and matrix, uniform distribution of clay may occur in matrix. The untreated nanoclay mainly montmorillonite (MMT) is comprised of silicate layer stacks. Each of stack is about 1 nm in thickness and is hydrophilic naturally. The silicate layer stacks are not appropriate for employment as filler in most commonly utilized epoxy resin which is hydrophobic [8]. Consequently, the montmorillonite clay is usually altered organically by an ion exchange process in which the cations are replaced by anions (as replacement of quaternary ammonium ions with long alkyl chains). By this route the spaces between clay layers increases which makes them organophilic. The epoxy resin intercalation in the preparation of epoxy/clay nanocomposites is strongly facilitated by ion exchange method. In order to fulfill the desired enhancement in mechanical features of these composites, it is essential to further separate the clay layers and homogenously disperse it throughout the polymer matrix during curing of epoxy/clay mixture. This process is also known as exfoliation [9, 10]. Moreover, the exfoliated nanostructure has been detected by small angle X-ray scattering (SAXS) and transmission electron microscopy (TEM). One of most significant triad of nanostructured carbon (fullerene and nanotube) is nanodiamond (ND) [11]. In the field of material science and engineering, ND powder synthesized from detonation technique has been extensively employed due to its outstanding thermal conductivity, thermal stability, excellent surface structures, remarkable electrical and mechanical properties as well as outstanding tribological features [12-14]. The surface groups may interact with polymer chain which result in generation of good adhesion between polymer matrix and nanodiamond [15]. The intra-gallery homo-polymerization reaction of epoxy may occur with the clay galleries to enhance the degree of exfoliation of epoxy-clay nanocomposite [16]. The hypothesis was supported by the studies on on organically modified MMT and bifunctional epoxy i.e. diglycidyl ether of bisphenol-A (DGEBA) [2, 3, 17]. In case of layered silicate nanocomposites based on tri-functional epoxy resin, triglycidyl para-amino phenol (TGAP) and MMT clay, two distinctive reactions were observed during isothermal curing reaction with 4,4-diamino diphenyl sulphone (DDS). The first was ascribed to the epoxy homopolymerisation reaction within the clay galleries, while the second was attributed to bulk cross-linking reaction in extra-gallery regions [18, 19]. The mechanism for improved exfoliation in epoxy/clay nanocomposite have been proposed using different preparation procedures: (i) incorporation of an initiator of cationic homopolymerisation within the clay galleries; (ii) pre-conditioning of the resin-clay mixture before the curing agent addition; and (iii) suitable selection of the isothermal cure temperature [20, 21]. In the present work, firstly montmorillonite (MMT) was intercalated with octadecylamine. Later, the intercalated MMT was used to form montmorillonite-nanodiamond filler (MMT-ND). Both the pristine nanodiamond as well as MMT-ND was used as filler to form composite with triglycidyl para-amino phenol employing diethyltoluene diamine as hardener. The cured samples were studied for morphology by atomic force microscopy. The relaxation temperature of cured systems was determined using dynamic mechanical thermal analysis. Further, the effect of different filler concentration on mechanical properties of epoxy resins was analyzed.
2. Experimental
2.1. Materials
- Triglycidyl para-amino phenol (TGAP), diethyltoluene diamine (DETDA), octadecylamine (97%), Na-montmorillonite, diamond nanopowder, hydrochloric acid (conc. HCl), and dimethylformamide were obtained from Aldrich.
2.2. Characterization Techniques
- Atomic force microscopy (AFM) images were obtained by MultiModeTMAFM (Digital Instruments). A Si tip was used with frequency of 300 kHz and spring constant of 40 N m-1. Rheometric scientific dynamic mechanical thermal analyzer (DMTA), bending cantilever, was used for the thermal and mechanical studies. The flexural properties were determined using ASTM standard D790M-93. Samples of 50×5×2 mm3 were run with a crosshead motion of 1.0 mm/min.
2.3. Intercalation of Montmorillonite
- 10 g montmorillonite was intercalated using 10g octadecylamine and 5 mL concentrated HCl in 100 mL distilled water. This solution was heated at 80°C. 20g of Na-montmorillonite was dispersed separately in distilled water. The dispersed clay was added to the solution of ammonium salt of octadecylamine and mixture was stirred for 6 h at 80°C. The precipitate was filtered, washed with water, and dried at 80°C for 24 h.
2.4. Formation of Nanodiamond Intercalated Montmorillonite (MMT-ND)
- 1 g intercalated MMT was dispersed in 100 mL of distilled water with sonication of 2 h. 1g nanodiamond was also separately dispersed in 50 mL of distilled water via sonication. Later, both the dispersions were mixed and sonicated for 2h, followed by reflux of 4h at 100°C. The mixture was filtered and washed repeatedly with absolute ethanol. The product was dried at 60°C (Fig. 1) [22].
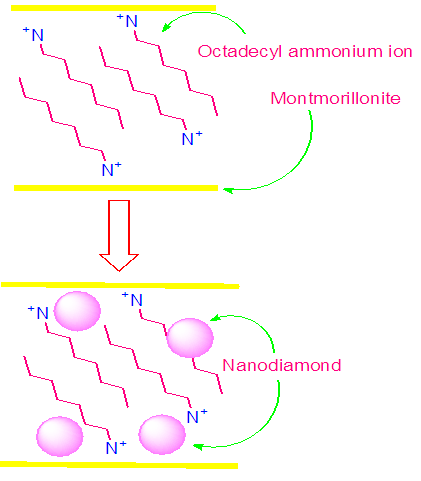 | Figure 1. Nanodiamond modification of montmorillonite |
2.5. Preparation of TGAP/ND and TGAP/MMT-ND Nanocomposite
- To 1g epoxy solution in DMF, desired amount of ND or MMT-ND (1-5 wt.%) nanofiller was dispersed and refluxed at 80°C. After reflux, the curing agent diethyltoluene diamine was added and mixed for 2h at 80°C. The epoxide to amine ratio was adjusted as 1:1 [23]. The reaction is shown in Fig. 2.
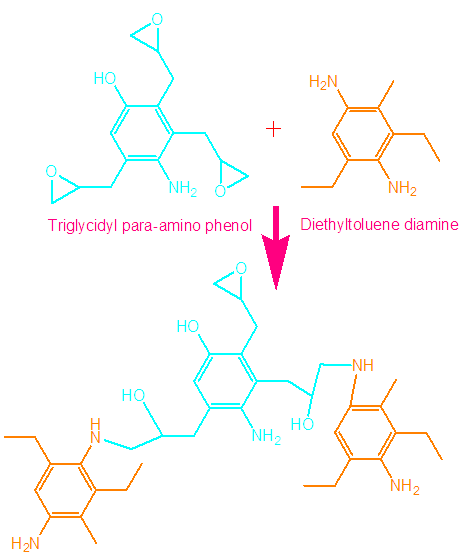 | Figure 2. Hardening reaction of epoxy |
3. Results and Discussion
3.1. Morphology Study
- AFM was used to investigate the nanocomposite surface [24, 25]. Fig. 3A &B shows AFM phase contrast images of TGAP/ND and TGAP/MMT-ND with 5 wt.% nanodiamond or hybrid filler. Both the images represent higher magnification of the examined areas. For TGAP/ND 5, AFM micrographs have shown dispersed nanodiamond particles in the matrix. The dispersion was found homogeneous in the micrographs. Some places have also shown the aggregated ND particles. The TGAP/MMT-ND with 5 wt.% nanofiller has shown uniform dispersion of MMT-ND nanofiller. The micrographs have also shown the dispersal of individual silicate layers. Few aggregated nanodiamond particles were also visible in the micrographs. Since this method investigated a small volume of bulk material, the overall morphology, however the overall morphology can be well predicted.
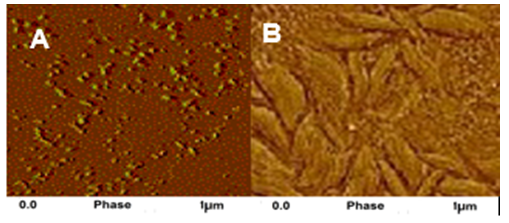 | Figure 3. AFM images of (A) TGAP/ND and (B) TGAP/MMT-ND with 5 wt.% filler |
3.2. Thermal Measurement
- As shown in Fig. 4, temperature location of β-relaxation peak for nanocomposite system decreased steadily with increasing ND and MMT-ND content in TGAP/ND and TGAP/MMT-ND. Moreover, the decrease in β-relaxation transition temperature was consistent. The β-relaxation temperature was decreased by 4-5°C in both the nanocomposites through the addition of 5 wt.% filler [26, 27]. It appears that the incorporation of nanofiller led to decrease in secondary relaxation temperature due to decrease in effective crosslinking density of epoxy resin. Generally, increase in crosslinking density in epoxy system may increase the relaxation temperature of the material [28]. It seems that the change in mobility of glassy state of epoxy resin due to nanofiller addition has influenced the β-relaxation temperature of the system. Moreover, the glass transition temperature of the composites was found in the range of 176-180°C [29].
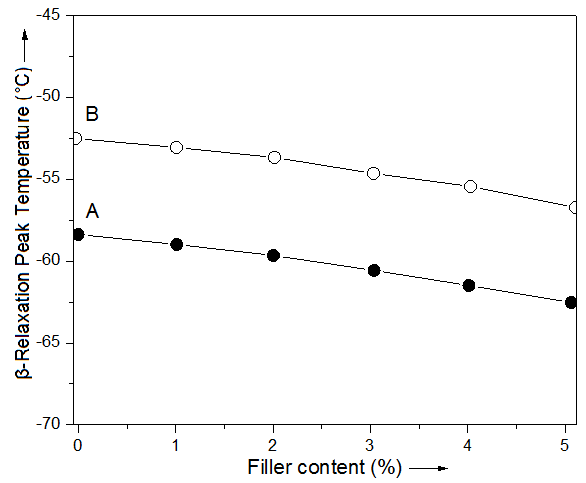 | Figure 4. β-Relaxation peak temperature determined from DMTA measurement of (A) TGAP/ND and (B) TGAP/MMT-ND with 5 wt.% nanofiller |
3.3. Mechanical Properties
- Modulus results for TGAP/ND and TGAP/MMT-ND with 1-5 wt.% nanofiller content are shown in Fig. 5. Both the nanocomposite series have shown increase in modulus with increasing nanofiller concentration. Fig. 5A shows increase in modulus from 1676 to 3074 MPa in TGAP/ND 1-5 system. Greater modulus increase was seen for TGAP/MMT-ND 1-5 from 2388 to 3408 MPa (Fig. 5B). The observed increase was probably due to the reinforcement effect of the hybrid nanofiller [30]. The normalized modulus was also calculated by dividing actual modulus with the modulus of unfilled system. Fig. 6 shows increase in modulus for TGAP/MMT-ND 1-5 systems relative to TGAP/ND 1-5 system. According to the results the reinforcement provided through nanodiamond exfoliation was because of shear deformation and stress transfer in the MMT particles.
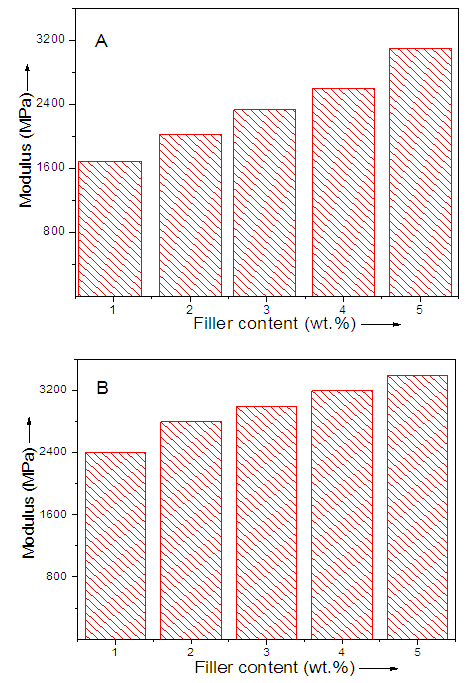 | Figure 5. Modulus of (A) TGAP/ND and (B) TGAP/MMT-ND with 1-5 wt.% filler |
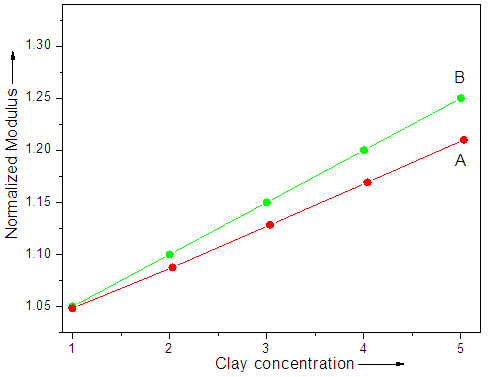 | Figure 6. Normalized modulus of (A) TGAP/ND and (B) TGAP/MMT-ND with 1-5 wt.% filler |
4. Conclusions
- Two systems of TGAP epoxy resin have been developed for comparative result analysis. Neat nanodiamond and ND intercalated montmorillonite was used as nanofiller. The montmorillonite was first intercalated with octadecylamine to increase the gallery spacing. Afterward, the intercalated MMT was used to form montmorillonite-nanodiamond filler. According to AFM, the MMT-ND morphology in epoxy matrix was dispersed nanodiamond in layered silicate structure. The relaxation temperature of TGAP systems was determined using dynamic mechanical thermal analysis. Further, the effect of different filler concentration on mechanical properties of epoxy resins was analyzed. The reduced β-relaxation temperature indicated lowering of crosslink density due to nanofiller particles. Normalized modulus and modulus of TGAP/ND 1-5 and TGAP/MMT-ND 1-5 were found to increase with nanofiller loading. The results have shown that the novel epoxy nanocomposites are attractive candidates for aircraft structuring.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML