-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Aerospace Sciences
p-ISSN: 2169-8872 e-ISSN: 2169-8899
2014; 3(2): 31-36
doi:10.5923/j.aerospace.20140302.01
Combustion Study of Composite Solid Propellants Containing Metal Phthalocyanines
Lalith V. Kakumanu1, Narendra Yadav1, Srinibas Karmakar2
1Department of Space Engineering and Rocketry, Birla Institute of Technology, Mesra, Ranchi, India
2Department of Aerospace Engineering, Indian Institute of Technology, Kharagpur, India
Correspondence to: Narendra Yadav, Department of Space Engineering and Rocketry, Birla Institute of Technology, Mesra, Ranchi, India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
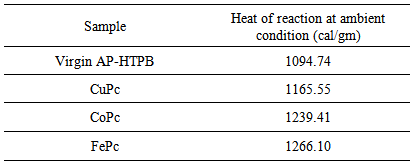
Transition metal Phthalocyanines have been used as burn rate modifiers for AP-HTPB composite solid propellants. An effort has been made to investigate the effect of Cu-Co-and Fe Phthalocyanines on the burn rate of composite propellants, as also on the combustion characteristics of such propellants. The burn rate has been determined in Crawford Strand Burner at 56 kg/cm2 pressure as also in low pressure burner setup at vacuum of 0.5 kg/cm2. Thermal analysis was performed using Thermo Gravimetric Analyzer (TGA), Differential Scanning Calorimeter (DSC) and heat of reaction has been measured by conventional Bomb Calorimeter. Scanning Electron Microscope (SEM) and Elemental Analyzer have been used for material characterization and possible composition of composite solid propellants. Metal Phthalocyanines have been found to influence the decomposition and to enhance the burn rate of the composite solid propellants.
Keywords: Composite Solid Propellant, Burn Rate, Combustion Characteristics, Metal Catalysts, Fuel-Binder
Cite this paper: Lalith V. Kakumanu, Narendra Yadav, Srinibas Karmakar, Combustion Study of Composite Solid Propellants Containing Metal Phthalocyanines, International Journal of Aerospace Sciences, Vol. 3 No. 2, 2014, pp. 31-36. doi: 10.5923/j.aerospace.20140302.01.
Article Outline
1. Introduction
- The design and operation of a rocket motor depends upon the burn rate of the propellant and the grain geometry of the propellant grain. The knowledge of burn rate thus becomes an important condition for a successful design of a solid rocket motor. The combustion chamber pressure has been found to be one of the most important design parameter, as the burn rate is exponentially dependent on the pressure. All the same, at a selected combustion chamber pressure, the designed burn rate of the propellant is feasible with the selected propellant not always available. The right selection of oxidizer particle size and oxidizer percentage does modify the burn rate; however the designed mechanical strength and other characteristics also get affected by these. Embedding metal wires or staples in the propellant also allows modification of burn rate; however the logistics and other complexities have desisted selection of this method as an attractive method of modification. The burn rate of a composite propellant can also be modified by incorporating burn rate modifier catalysts. In fact, this method has been found to be the best and most effective method. Extensive research has been advanced in search of an effective catalyst, and metal Phthalocyanines happen to be one of such catalysts. The MPcs are candidate materials in high technology applications like molecular electronics, liquid crystal displays, gas sensors and organic laser materials. Their useful properties are attributed to their efficient electron transfer abilities. The central cavity of MPc is known to be capable of accommodating 63 different elemental ions. The functions of MPc are almost universally based on electron transfer reactions because of the 18 π electron conjugated ring system found in their molecular structure [1]. They have excellent stability against heat, light and air- moisture, and found to be effective in many especially designed catalytic reactions [2]. Bill, et al. [3] have suggested their uses as radiation absorbers. Henry [4] has reported their activeness for catalyzing oxidation reactions. According to Hronec, et al. [5] metal Phthalocyanines act towards electrode reduction of oxygen. Wheatley et al. [6] reported improvement of thermal stability and enhancement of burn rate with decreased pressure exponent by adding copper Phthalocyanines (CuPc). Lundstrom et al. [7] used CuPc as a low pressure combustion chamber. Fong, et al. [8] studied the effect of CuPc in AP-HTPB based propellant and reported increase of the pressure range of combustion and enhancement of burn rate of DDI cured HTPB. Bain and Rudy [9] reported the tendency towards combustion instability by addition of CuPC. Kandaz, et al. [10] as well as Bolker and Salker [11] conducted studies with Cobalt Phthalocyanine (CoPc). Zheng and Zhang [12] found that Iron Phthalocyanine (FePc) has large ionization potential and better di-oxygen binding capability making them a worthy catalyst. Eileen, et al. [13] showed high oxygen reduction activity by using FePc. In the light of this, the background investigation has been carried out using CuPc, CoPc and FePc as catalysts in AP-HTPB composite solid propellants. Combustion study of solid propellants containing various contents of MPcs have been investigated and an effort has been made to obtain detailed experimental data on the burning characteristics of AP-HTPB based solid propellants to understand the role of MPcs as burn rate modifier.
2. Experimental
2.1. Materials
- Cobalt (II) Phthalocyanines (CoPc), 97%, Iron (II) Phthalocyanines (FePc), 90% and Copper (II) Phthalocyanines (CuPc), 95% were purchased from Alfa Aesar India Ltd. HTPB of R45M grade was supplied by kind request from Vikram Sarabhai Space Center (VSSC), India. Ammonium Perchlorate (AP) was purchased from Tamilnadu Chlorate, India Ltd. Bis(2-ethylhexyl) adipate (BEHA) of 99%, and Tolylene-2,4-diisocyanate (TDI) of 95% pure were procured from Sigma-Aldrich. Glycerol of analytical grade from CDH India Ltd. was used for propellant formulation.
2.2. Processing of Solid Propellants
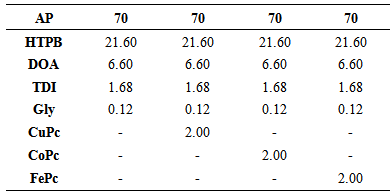
- The composite solid propellants (CSPs) were formulated by using HTPB fuel binder and AP as a solid oxidizer. AP composition was taking uniform, 70 percent, in all such propellants samples. The average particle sizes of AP were 44µ and 250µ in the ratio of 3:1 in the propellant samples. BEHA was used as a plasticizer. The products made with it have good softness and excellent viscoelastic property. It was dehumidified before the usage. Toluene Di-Isocyanate (TDI) was used as a curing agent because it reacts with hydroxyl groups in HTPB to form poly urethane linkages. Besides this, Glycerol was used as a cross-linking agent to provide necessary branching for the fuel binder. The CuPc, CoPc and FePc have been used as burn rate modifier catalysts. The composition is given below (Table 1) in weight percentages, catalysts are added extra. The requisite quantity of ingredients was weighed properly, thoroughly mixed, casted and cured for 6 days at 60 ±1°C in a hot air oven and cut into strands for the experimentation.
|
2.3. Instrumentals
- Thermal decomposition process of the propellants was carried out with the help of Shimadzu DTG-60 Thermo Gravimetric Analyzer (TGA) and differential thermal analysis (DTA) techniques whereas, TA Instrument USA-Q10 differential scanning calorimeter (DSC) was used to characterize the propellant samples at heating rate of 10°C/min, in the temperature range between 30 to 300°C. The surface examination of propellant samples were performed with the help of scanning electron microscopy (SEM) (Model: Jeol-JSM 6390 LV, Japan) with the following specification; accelerating voltage (10 KeV), secondary electron image mode, and with 20 mm working distance. The test samples were mounted on aluminum stub and coated with gold metal to avoid electrical charging during analysis. Heat of reactions of prepared propellant samples was determined with the help of Digital Bomb Calorimeter (ARICO, India) at atmospheric pressure and inert atmosphere. Linear burn rates of solid propellants were measured by using standard Crawford Strand Burner and Low Vacuum combustion setup respectively.
3. Results and Discussion
3.1. Thermal Properties
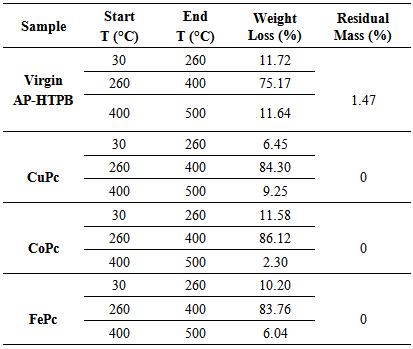
- Thermal decomposition characteristics of solid propellants (as shown Figure 1) with and without catalysts (MPcs) are investigated by thermo gravimetric analysis (TGA). The percentage weight loss and residual mass percent of each sample between 30 to 500°C have been evaluated and properly presented in Table 2. The decomposition of Virgin AP-HTPB sample was found to be three staged, but on addition of 2% of CuPc, CoPc and FePc. The thermal decomposition has become a two staged decomposition with a sudden mass loss, thus increasing the decomposition rate. The decomposition patterns indicated that primarily depolymerization and partial decomposition of crosslinked HTPB matrixes. Certain products made by self-cyclization at given temperature which may decompose comparatively at higher temperature range.
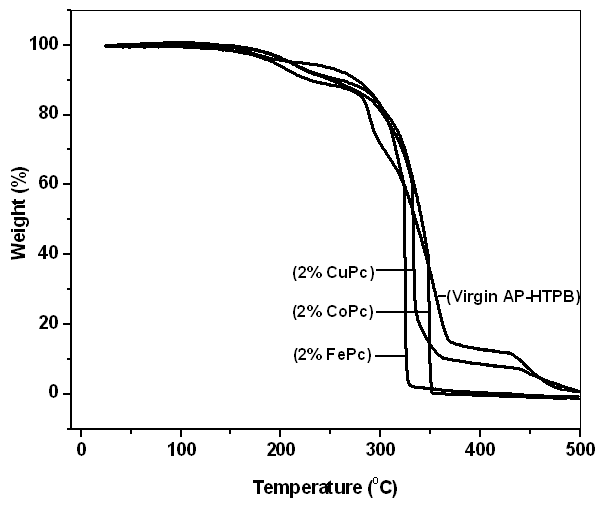 | Figure 1. TGA curves for propellant samples |
|
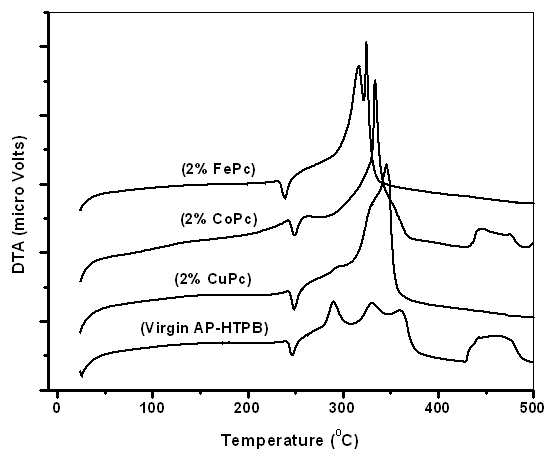 | Figure 2. DTA thermograms of propellant samples |
|
|
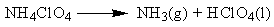 | (1) |
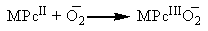 | (2) |
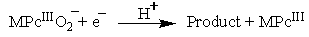 | (3) |
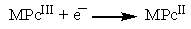 | (4) |
|
3.2. Surface Characterization
- SEM image of virgin-AP HTPB propellant (Figure 3) showed that AP and HTPB have blended well with each other and have formed a uniform composite matrix. On the addition of 2% CuPc, the texture of propellants become irregular but dispersion of MPcs molecules was found to uniform throughout the surfaces. AP particles were also seen encapsulating with HTPB which leads to development of cubic crystal rather than spherical shape. SEM images evidence the morphology of the material and the absence of micro-voids and dewetting phenomena. We notice that on the addition of 2% CoPc, the mixture is not homogeneous as compared to the virgin AP-HTPB mixture but the consistency is better than the sample of 2% CuPc. Small amounts of AP-HTPB chunks coated with CoPc can be seen in SEM image.
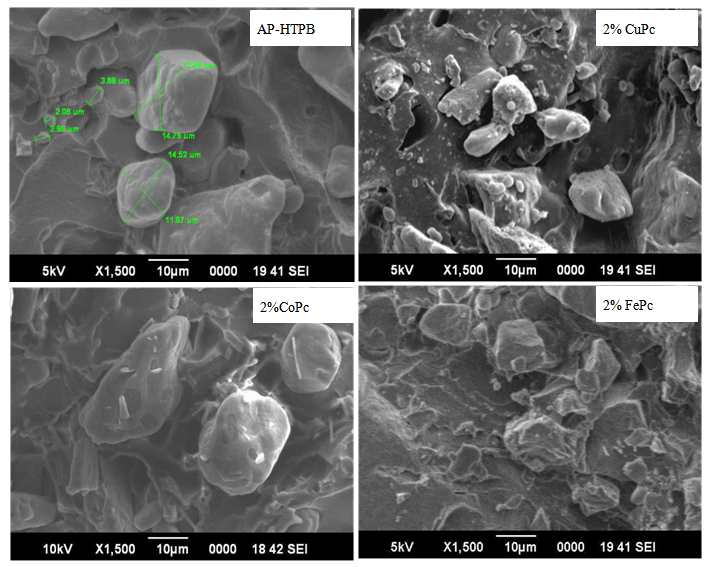 | Figure 3. Surface morphologies of solid propellant samples |
3.3. Elemental Analysis
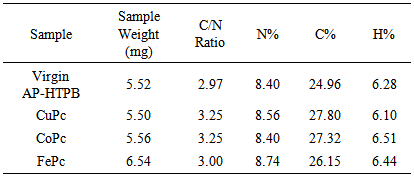
- In this analysis, each sample was combusted at high temperatures in oxygen rich environment and all the combustion products were swept out of the combustion chamber by an inert carrier gas (helium). These products were passed over a heated high purity copper which removes unconsumed oxygen and converts nitrogen oxides into nitrogen gas. These gases were passed through a thermal conductivity detector which detects the amount of Carbon, Hydrogen, Nitrogen and Sulfur present in the compound. Table 6 gives the results of the CHNS Analysis. It can be observed that the C/N Ratio has increased on the addition of Metal Phthalocyanines. This phenomenon has to be studied further to get an insight into the actual combustion process that is taking place.
|
3.4. Burning Characteristics
- The burn rate studies at high operating pressures were investigated using a Crawford Strand Burner. The propellant strands were electrically ignited and the time taken for 4 cm length of the strand to combust was recorded and then burn rate was calculated. The firing vessel was filled with nitrogen gas at 56 kg/cm2 to simulate the operating chamber pressure conditions. From Figure 4 we notice that there is an increase in the burn rate of the propellants on the addition of metal phthalocyanines. Of all the propellant samples, with FePc as catalyst have the highest burning rate in comparison with others at high pressure. Similar effect with FePc was also observed by Fong et al. [8]. This increase in burn rate could be because of the MPc addition. The addition of MPc increases the O2 reduction activity which is controlled by the MPcII/MPcIII redox couple as quoted in the mechanism in the earlier section.
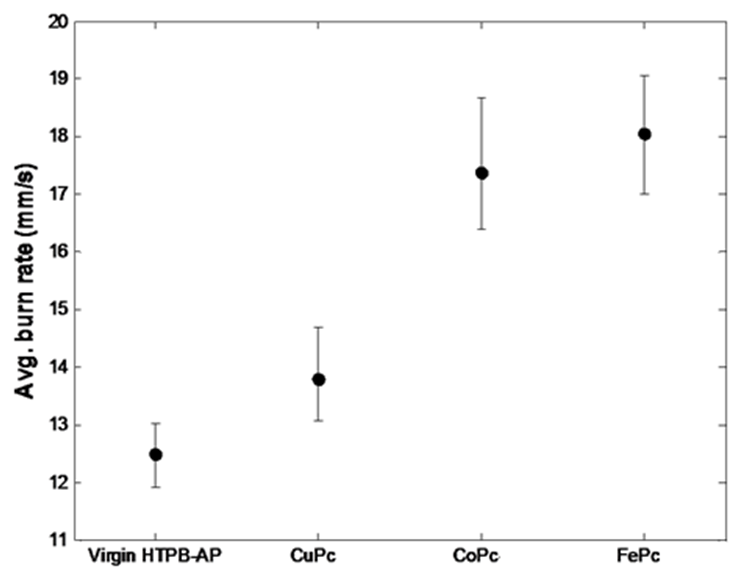 | Figure 4. Plot of burn rate of propellants at high Pressure |
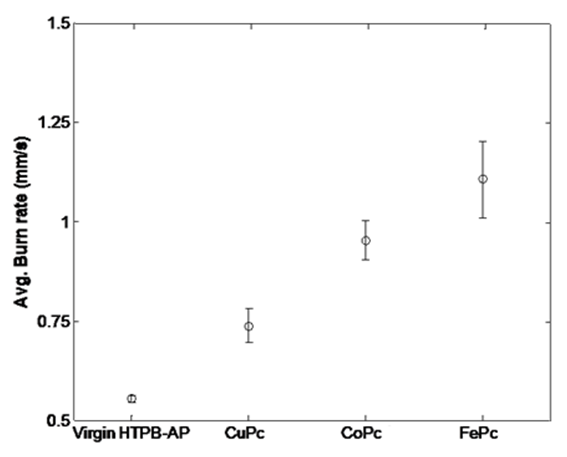 | Figure 5. Plot of burn rate of propellants at low pressure |
4. Conclusions
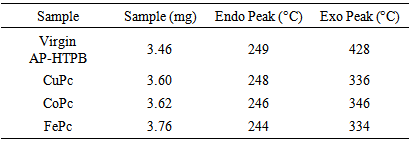
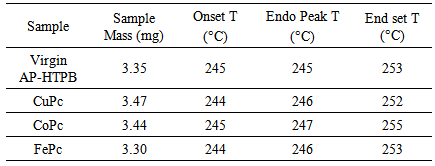
- The following conclusions can be drawn from the present investigations on the combustion characteristics of AP-HTPB composite solid rocket propellants with and without metal Phthalocyanines as burn rate modifiers.All the samples except the one with 2% CuPc blendedwell with the AP-HTPB slurry. This is because CuPc coats the slurry as soon as it comes in contact with the slurry and does not mix thoroughly to get a consistent mixture as compared to the other metal phthalocyanines used.On addition of 2% of CuPc, CoPc and FePc, the thermal decomposition of virgin AP-HTPB which was found to be a three staged decomposition has become a two staged decomposition with a sudden mass loss, thus increasing the decomposition rate. Addition of metal phthalocyanines does not show appreciable reduction in onset, endset and endothermic peak temperatures; however, a significant reduction in the exothermic peak temperature has been observed.The presence of metal phthalocyanines in the AP-HTPB sample has increased the heat of reaction of the composite solid rocket propellant which means the catalysts can lead to more completeness of the combustion of AP-HTPB propellant.The addition of metal phthalocyanines has been found to increase the burning rate of the propellants in both high pressure and low vacuum conditions. This is beneficial because the enhanced burning rate can attribute to better performance of the rocket.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML