-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Computing
p-ISSN: 2163-2944 e-ISSN: 2163-2979
2014; 4(2): 41-54
doi:10.5923/j.ac.20140402.02
Automaticand Accurate Segmentation of Gridded cDNA Microarray Images Using Different Methods
Islam Fouad1, Mai Mabrouk2, Amr Sharawy3
1College of Applied Medical Sciences, SALMAN Bin ABDUL-AZIZ University, Kharj, KSA
2Biomedical Engineering, MUST University, Egypt
3Biomedical Engineering, Cairo University, Giza, Egypt
Correspondence to: Mai Mabrouk, Biomedical Engineering, MUST University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
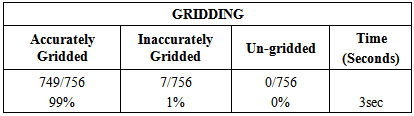
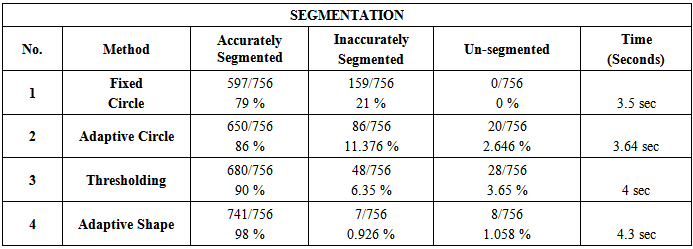
Due to the vast success of bioengineering techniques, a series of large scale analysis tools has been developed to discover the functional organization of cells. Among them, cDNA microarray has emerged as a powerful technology that enables biologists to cDNA microarray technology has enabled biologists to study thousands of genes simultaneously within an entire organism, and thus obtain a better understanding of the gene interaction and regulation mechanisms involved. The analysis of DNA microarray image consists of several steps; gridding, segmentation, and quantification that can significantly deteriorate the quality of gene expression in formation, and hence decrease our confidence in any derived research results. Thus, microarray data processing steps become critical for performing optimal microarray data analysis and deriving meaningful biological information from microarray images. Gridding; the first processing step in microarray image analysis, is to allocate each spot of the array inside a distinct window. The second step which is highly affected by gridding is segmentation. It is the process, by which each individual cell in the grid must be selected to determine the spot signal and to estimate the background hybridization. In this paper, an accurate and fully automated gridding method is applied to prepare the image for the Segmentation step. For segmenting the microarray image four segmentation methods are explored; “fixed circle”, “adaptive circle”, “thresholding”, and “adaptive shape” segmentation. By comparing the results of segmentation, it was found that the “adaptive shape segmentation method” can segment noisy microarray images correctly, gives high accuracy results and minimal processing time, and can be applied to various types of noisy microarray images.
Keywords: Noisy Microarray Image, Gene Expression, Analysis of DNA Microarray Image, Gridding, Segmentation
Cite this paper: Islam Fouad, Mai Mabrouk, Amr Sharawy, Automaticand Accurate Segmentation of Gridded cDNA Microarray Images Using Different Methods, Advances in Computing, Vol. 4 No. 2, 2014, pp. 41-54. doi: 10.5923/j.ac.20140402.02.
Article Outline
1. Introduction
- Microarray technology came on time to cover the need to monitor in parallel all the DNA sequences and to have the adequate sensibility to detect the variation of gene expression. There may be tens of thousands of spots on an array. Each spot contains tens of millions of identical DNA molecules with lengths from tens to hundreds of nucleotides. Afterwards, the microarray slide is exposed to a set of labeled cDNA samples, which are derived from tissue of interest. With the completion of hybridization reaction, the amount of the target that bounds to each sample is measured with the aid of image capturing devices and computer technology. The measurement is based on the intensity of the spot. There are three basic steps in the processing of microarray image [1]; gridding, segmentation, and quantification.The first step is gridding; to assign coordinates to every element of the spot array. Gridding is the primary task of cDNA microarray image analysis; therefore it is a prerequisite for follow-up to microarray analysis.Major work has been presented in the domain of microarray image gridding. Reference [2] described a semi-automatic system which mainly focused on the problem of finding an individual spot with high accuracy. Reference [3] introduced a technique using morphological operators to perform automatic gridding procedures for sub grids and spots. Reference [4] described a system for microarray gridding and quantitative analysis that imposed different kinds of restrictions on the print layout. That method required the rows and columns of all grids to be strictly aligned. Antoniol and Ceccarelli 2004 applied the markov random field approach which required the user’s input of the size of the spot, and the number of rows and columns. Reference [5] used a predefined image filter to grid the sub-array image.During the recent years, there have been methods and software packages available that deal with one or a few of the aforementioned problems, such as ScanAlyze, 19 Spot (Buckly 2000), and GenePix Pro6 (Axon Instruments Inc. 2004). However, they all require a certain level of human intervention for achieving the desired accuracy, which imposes a big burden on the biologists who use microarrays in their research [6].Image segmentation is the process of distinguishing objects from their background [7]. It is usually the first step in vision systems, and is the basis for further processing such as description or recognition. The goal of segmentation is to extract important features from images. Segmentation of an image can also be seen, in practice, as the classification of each image pixel to be assigned to one of the image compositions.Different segmentation methods have been presented include the dynamic system modeling based approach [8] performs pixel clustering operations in a parallel manner to speed-up the segmentation process. The cellular neural network scheme [9, 10] segments the spots by performing a number of operations such as background clean-up, grid analysis, irregular spot elimination, and intensity analysis. The morphology based approach [11] uses a series of optional steps to segment the microarray image. The combination of Markov random field based grid segmentation and active contour modeling constitutes an approach suitable for spot detection and segmentation [12]. The two-stage clustering based approach [13] is comprised of spots boundaries adjusting and intensity-based partitioning operations. The use of adaptive thresholding and statistical intensity modeling is the base for some segmentation schemes [14], whereas another approach [15] uses a seeded region growing algorithm to identify spots of different shapes and sizes. Histogram and thresholding operations were used to classify microarray image samples into either foreground (spots) or background pixels [16].One of the key steps in extracting information from a well gridded microarray image is the segmentation whose aim is to identify which pixels within an image represent which gene. This task is greatly complicated by noise within the image and a wide degree of variation in the values of the pixels belonging to a typical spot. An effective fully automated gridding technique followed by a comparison between four different segmentation methods is presented on a noisy microarray image, with high accuracy. The paper is organized as follows: a brief introduction is presented in this section, section 2 presents the used materials, section 3 summarizes the proposed gridding and segmentation methods for various cDNA microarray noisy images and section 4 discusses the results of the applied algorithms on microarray data set image. Conclusions are presented in section 5.
2. Materials
- Different images have been selected from two different data sets, to test the performance of the proposed methods. They have different scanning resolutions, and different noise types, in order to study the flexibility of the proposed methods to detect spots with different sizes and features.The images are stored in TIFF files with 16-bit gray level depth. A chosen microarray image with various kinds of artifacts or noises is drawn from Princeton University Microarray (PUMA) database [17]. The image includes thirty two sub-grids presenting acute lymphoblastic leukemia tissues (PUMA Experiment ID: 10223). The slide name is (shae082) and it is a cDNA microarrays spotted by a total of 24192 genes.MATLAB [18] is used for data analysis and technical computing, as it is a high performance and powerful tool. MATLAB version is 7.11.0.584 (R2012b) on a windows 7 platform. The P.C used has a processor: Intel (R) Core (TM)i5 – 2.27 GHz. CPU with 2GB of memory.
3. Methods
3.1. Microarray Image Gridding
- A gridding method using projection technique is proposed. This method is useful to eliminate various types of noise occurred in microarray images. Preprocessing microarray image is required before applying the gridding step, in order to remove noise which has gray values on the black background using Global Background Noise Correction. It can be implemented through a number of steps staring by getting the median intensity value of the image, then check each pixel value in the image, and compare this value with the obtained median value. If the pixel intensity value is less than the median value, we set it to zero. Otherwise, the pixel value is remained as shown in figure 1.
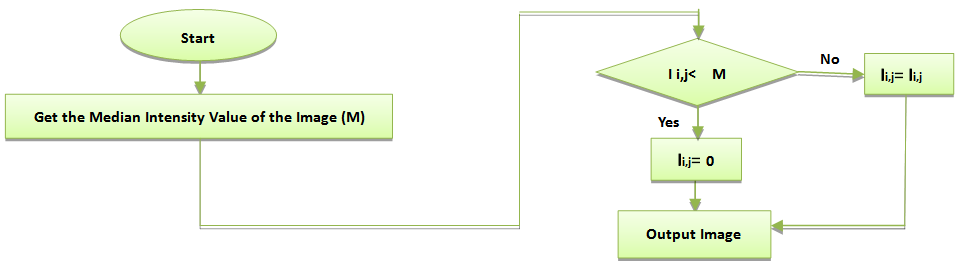 | Figure 1. Flowchart of Global Background Noise Correction |
 row and the
row and the  column. Because of the low-intensity features that are not well distinguishable from the background in most of the microarray images, it's important to develop a new method to improve the contrast between the foreground (spots) and the background using histogram equalization [18-20]. Unfortunately, an additive noise (small white spots) appeared on the background which can be eliminated using wiener filter [18-20]. In order to remove the large flare noise; at first the erosion operator with a structuring element (In experiment se = 7) is applied to remove foreground spots [18, 21], then apply image reconstruction to the result background [18, 19]. An image with less noise is obtained by subtracting resulted background from original image. This is mainly to remove large flare noise. After that, morphological opening with a structuring element (In experiment se = 4) is applied to remove small spikes in the image and at last apply image reconstruct again to the obtained image as shown in figure 2.
column. Because of the low-intensity features that are not well distinguishable from the background in most of the microarray images, it's important to develop a new method to improve the contrast between the foreground (spots) and the background using histogram equalization [18-20]. Unfortunately, an additive noise (small white spots) appeared on the background which can be eliminated using wiener filter [18-20]. In order to remove the large flare noise; at first the erosion operator with a structuring element (In experiment se = 7) is applied to remove foreground spots [18, 21], then apply image reconstruction to the result background [18, 19]. An image with less noise is obtained by subtracting resulted background from original image. This is mainly to remove large flare noise. After that, morphological opening with a structuring element (In experiment se = 4) is applied to remove small spikes in the image and at last apply image reconstruct again to the obtained image as shown in figure 2.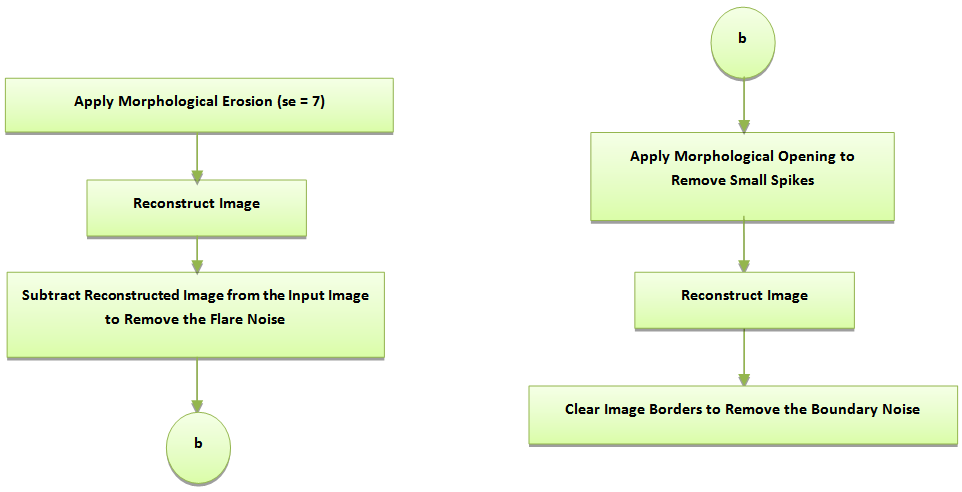 | Figure 2. Flowchart of Flare Noise Removal |
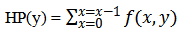 | (1) |
3.2. Segmentation
- Once grids have been placed, discrimination between areas that are considered the spotsignal and areas that are considered the background signal must be carried out. The process, by which each individual cell in the grid must be selected to determine the spot signaland to estimate the background hybridization, is called segmentation. That information will be put towards a quantitative measurement at each cell. There are four presented approaches for segmentation; fixed circle segmentation, adaptive circle segmentation, thresholding segmentation, and adaptive shape segmentation.• Fixed Circle SegmentationFixed circle segmentation method assigns all the spots the same size and shape. It uses a constant-diameter circle as the shape of all the spots in the image. The proposed fixed circle segmentation technique is presented as in the following steps:1. Input the gridded image.2. Get the region of interest (R.O.I.).3. Let I = ith row in the R.O.I.
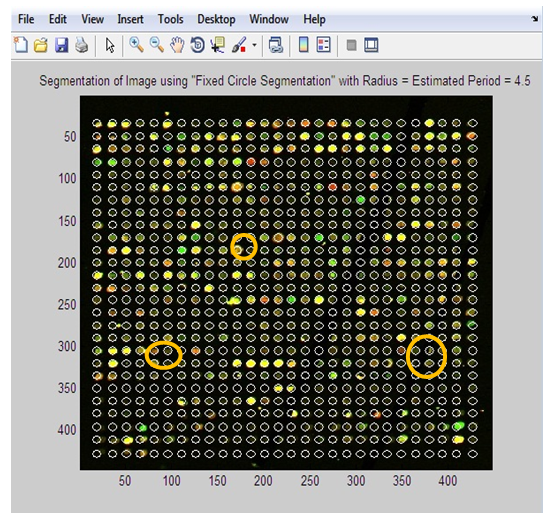
 I = 1 to end of R.O.I.4. For each I, we calculate the center of its corresponding boundary box.5. Draw a circle with the obtained center and the obtained estimated period (distance between two adjacent spots).• Adaptive Circle SegmentationAdaptive circle segmentation considers the shape of each spot as a circle, where the center and diameter of the circle are estimated for each spot. It involves two steps. First, the center of each spot needs to be estimated. Second, the diameter of the circle has to be adjusted. Adaptive Circle Segmentation method is shown in figure 3.
I = 1 to end of R.O.I.4. For each I, we calculate the center of its corresponding boundary box.5. Draw a circle with the obtained center and the obtained estimated period (distance between two adjacent spots).• Adaptive Circle SegmentationAdaptive circle segmentation considers the shape of each spot as a circle, where the center and diameter of the circle are estimated for each spot. It involves two steps. First, the center of each spot needs to be estimated. Second, the diameter of the circle has to be adjusted. Adaptive Circle Segmentation method is shown in figure 3.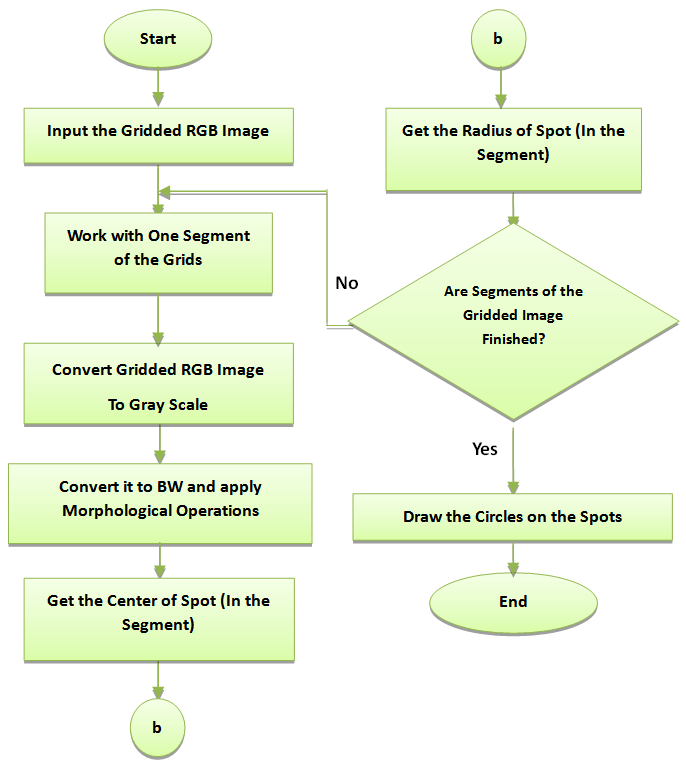 | Figure 3. Flowchart of Adaptive Circle Segmentation |
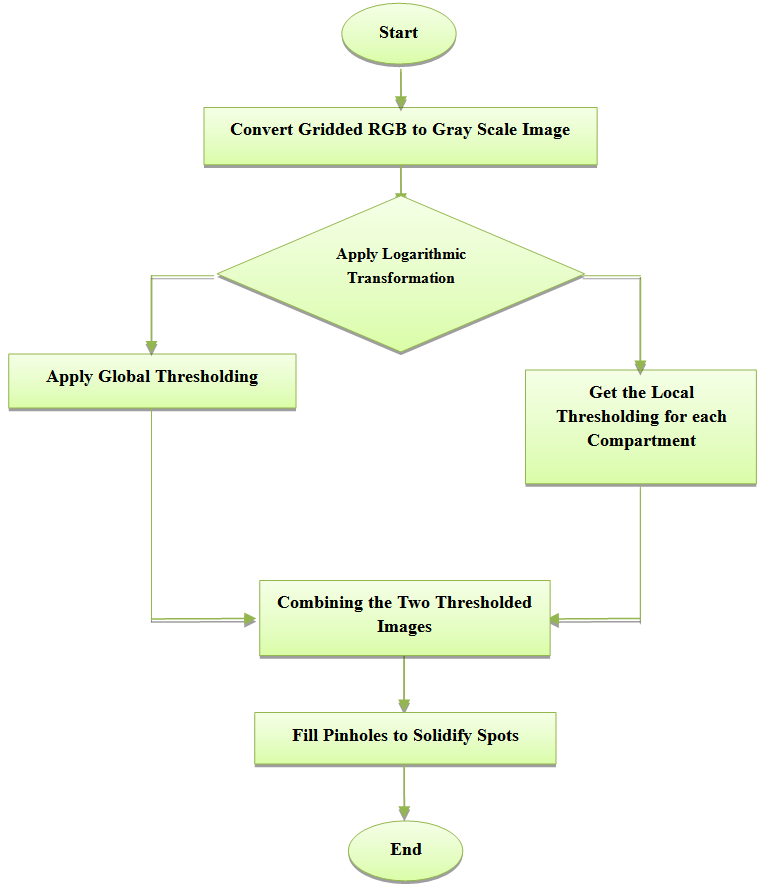 | Figure 4. Flowchart of Thresholding Segmentation |
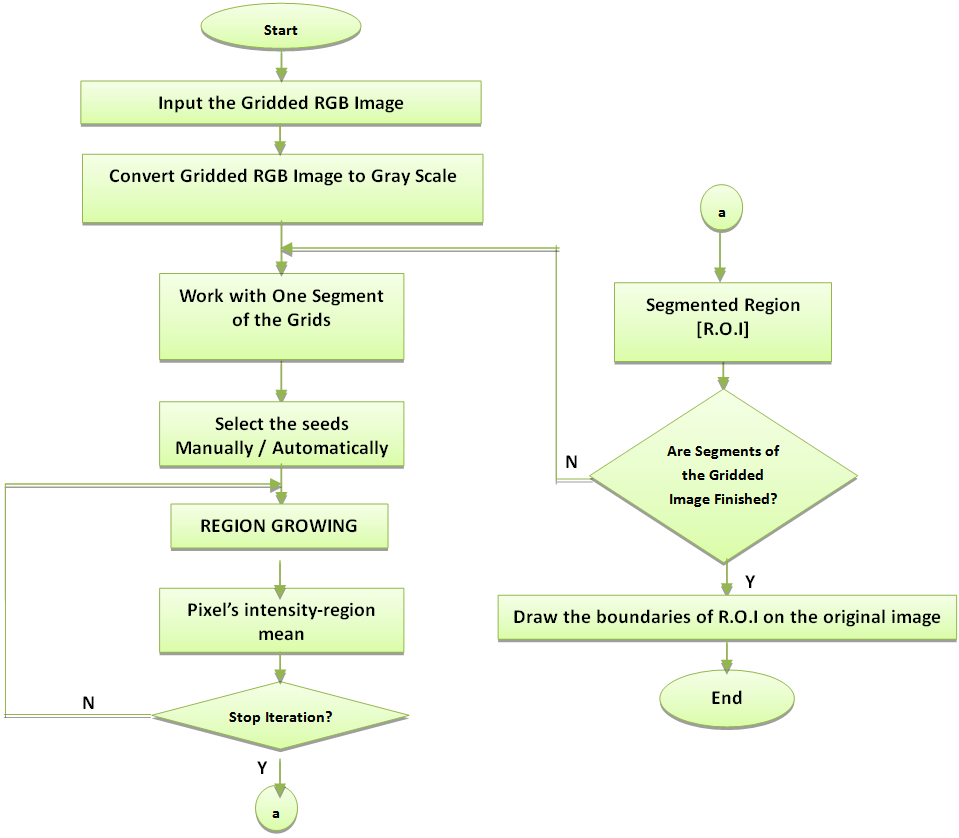 | Figure 5. Flowchart of S.R.G. Segmentation |
4. Results and Discussion
- Anautomatic gridding method based on noise removal and four different segmentation methods; Fixed Circle, Adaptive Circle, Thresholding, and Adaptive Shape Segmentation were presented.These presented methods are tested on a number of noisy microarray images drawn from Princeton University Microarray (PUMA) database. The results of applying these segmentation methods on a microarray image are shown in this section. The chosen noisy microarray image includes thirty two sub-grids (PUMA Experiment ID: 10223) and it is spotted by a total of 24192 genes. To test the efficiency of the proposed methods, a sub-array in the fourth row and the second column was cropped as shown in figure 6.
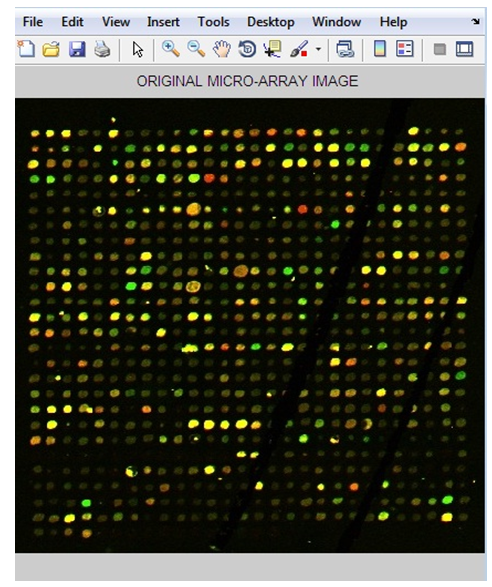 | Figure 6. The Original Microarray Image |
4.1. Gridding
- Figure 7 shows the detailed results after applying the proposed gridding method.
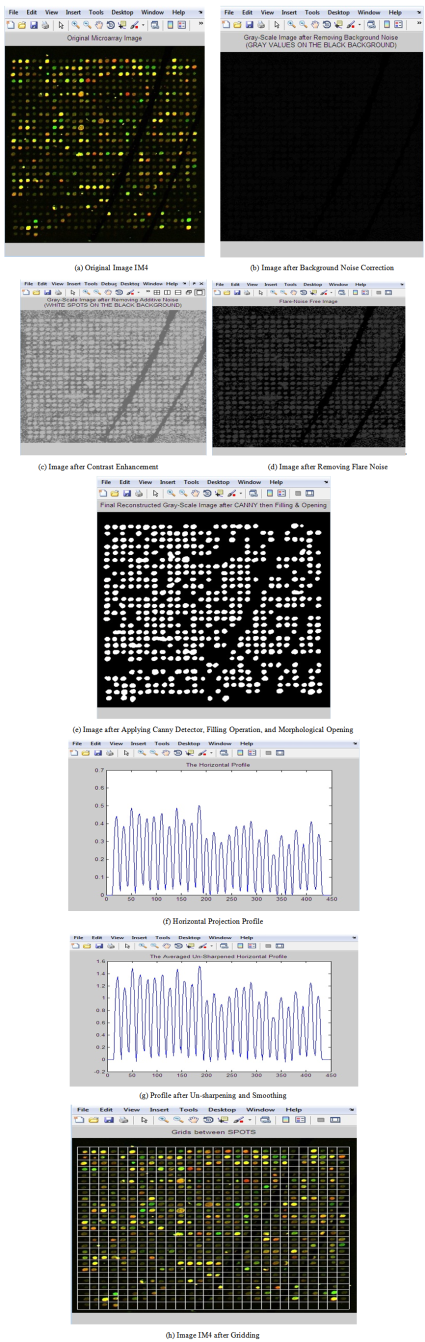 | Figure 7. Steps of applying the proposed gridding technique on original image |
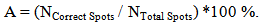 | (2) |
|
4.2. Segmentation
- • Fixed Circle SegmentationIt was obviously shown that the fixed circle method clearly cannot satisfy the needs. Figure 8 shows the resulting image after applying the fixed circle approach. We can see that some regions within the high intensity areas (spots) are left out of the foreground, and some regions within the low intensity areas (background) are included in the foreground regions. That is because of the fixed diameter of all the drawn circles despite the variation in the diameters of the spots in the same image.
 | Figure 8. Image after Applying “Fixed Circle Segmentation” Method |
 | Figure 9. Image after Applying “Adaptive Circle Segmentation” Method |
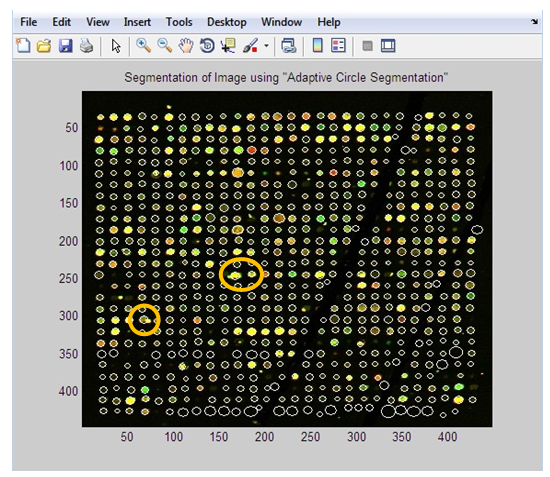
 | Figure 10. Steps of applying “Thresholding Segmentation” technique on original image |
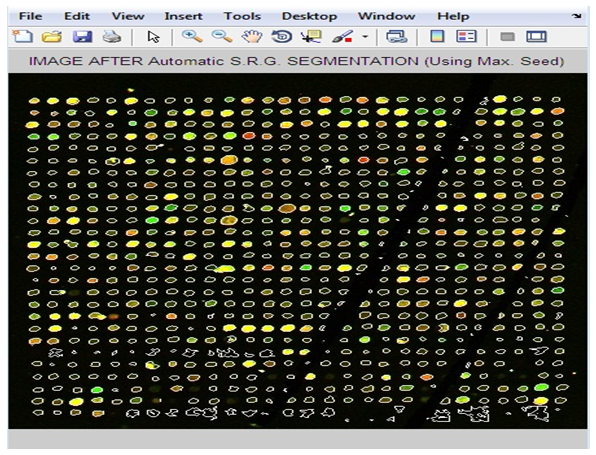 | Figure 11. Image after Applying “Adaptive Shape Segmentation” Method |
|
5. Conclusions
- The development of biomedical research has been led by the increasing knowledge as well as new advances in technology. Traditionally, researchers were able to investigate a small number of genes at a time by using the available techniques back then. DNA microarray technology has enabled biologists to study all the genes within an entire organism to obtain a global view of genes’ interaction and regulation. This technology has a great potential in obtaining a deep understanding of the functional organization of cells. The emergence of this technology allows the researchers to tackle difficult problems and reveal promising solutions in many fields.In this respect, our research based heavily on the requirement for areliable yet time efficient automated method. One of the key steps in extracting information from a well-gridded microarray image is the segmentation whose aim is to identify which pixels within an image represent which gene. This task is greatly complicated by noise within the image and a wide degree of variation in the values of the pixels belonging to a typical spot.This work emphasizes the impact of well-gridded microarray image segmentation. The proposed gridding method depends on projection and applicable to various types of noisy microarray images. It gives very good results with accuracy 99%. In the segmentation stage, four segmentation methods have been presented; “fixed circle segmentation”, “adaptive circle segmentation”, “thresholding segmentation”, and “adaptive shape segmentation” methods. When the four proposed methods sere compared, it was found that the first two methods are shape-based segmentation methods. Fixed circle segmentation cannot segment clearly all types of images; when the spots size varies in the image, it will work badly. While the adaptive circle segmentation method achieves better results for circle-shaped spots. The other two segmentation methods; “adaptive shape segmentation” and “thresholding segmentation” gives better results. When using “thresholding segmentation” to separate background from foreground in microarray images, the current methods cannot deal well enough with weak spots. So, it was very clear that “adaptive shape segmentation” gives better results using a maximum seed with accuracy 98%.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML