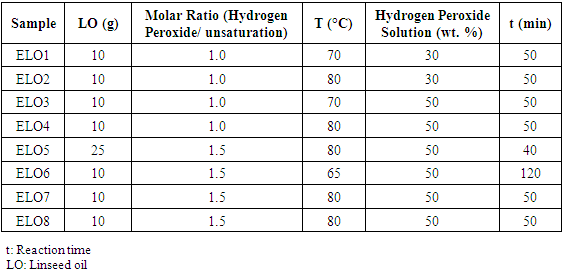
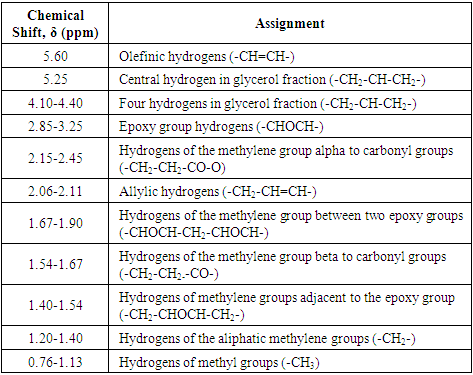
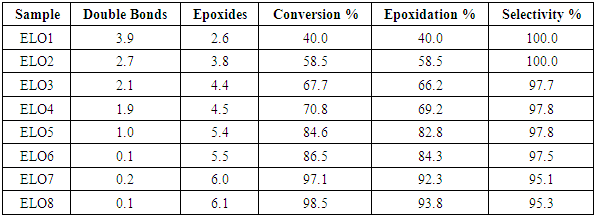
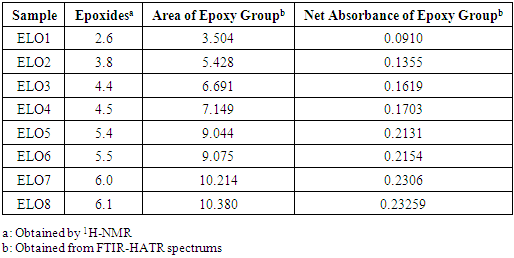
| [1] | P. Karmalm, T. Hjertberg, A. Jansson, and R. Dahl, “Thermal stability of poly(vinyl chloride) with epoxidised soybean oil as primary plasticizer,” Polym. Degrad. Stab., vol. 94, no. 12, pp. 2275–2281, 2009. |
| [2] | Y. B. Tee, R. A. Talib, K. Abdan, N. L. Chin, R. K. Basha, and K. F. Md Yunos, “Comparative study of chemical, mechanical, thermal, and barrier properties of poly(lactic acid) plasticized with epoxidized soybean oil and epoxidized palm oil,” BioResources, vol. 11, no. 1, pp. 1518–1540, 2016. |
| [3] | D. Garcia-Garcia, A. Carbonell-Verdu, M. P. Arrieta, J. López-Martínez, and M. D. Samper, “Improvement of PLA film ductility by plasticization with epoxidized karanja oil,” Polym. Degrad. Stab., vol. 179, p. 109259, Sep. 2020. |
| [4] | E. A. Baroncini, S. Kumar Yadav, G. R. Palmese, and J. F. Stanzione III, “Recent advances in bio-based epoxy resins and bio-based epoxy curing agents,” J. Appl. Polym. Sci., vol. 133, no. 45, pp. 1–19, Dec. 2016. |
| [5] | Y. Chen, Z. Xi, and L. Zhao, “New bio-based polymeric thermosets synthesized by ring-opening polymerization of epoxidized soybean oil with a green curing agent,” Eur. Polym. J., vol. 84, pp. 435–447, 2016. |
| [6] | J. M. Pin, N. Sbirrazzuoli, and A. Mija, “From epoxidized linseed oil to bioresin: An overall approach of epoxy/anhydride cross-linking,” ChemSusChem, vol. 8, no. 7, pp. 1232–1243, 2015. |
| [7] | Y. Y. Liu, J. He, Y. D. Li, X. L. Zhao, and J. B. Zeng, “Biobased, reprocessable and weldable epoxy vitrimers from epoxidized soybean oil,” Ind. Crops Prod., vol. 153, no. May, p. 112576, 2020. |
| [8] | A. Sammaiah, K. V. Padmaja, and R. B. N. Prasad, “Synthesis of epoxy jatropha oil and its evaluation for lubricant properties,” J. Oleo Sci., vol. 63, no. 6, pp. 637–643, 2014. |
| [9] | G. Gorla, S. M. Kour, K. V. Padmaja, M. S. L. Karuna, and R. B. N. Prasad, “Preparation and properties of lubricant base stocks from epoxidized karanja oil and its alkyl esters,” Ind. Eng. Chem. Res., vol. 52, no. 47, pp. 16598–16605, 2013. |
| [10] | R. Tesser, V. Russo, R. Turco, R. Vitiello, and M. Di Serio, “Bio-lubricants synthesis from the epoxidized oil promoted by clays: Kinetic modelling,” Chem. Eng. Sci., vol. 214, p. 115445, 2020. |
| [11] | E. Albarrán-Preza, D. Corona-Becerril, E. Vigueras-Santiago, and S. Hernández-López, “Sweet polymers: Synthesis and characterization of xylitol-based epoxidized linseed oil resins,” Eur. Polym. J., vol. 75, pp. 539–551, 2016. |
| [12] | P. Alagi, Y. J. Choi, J. Seog, and S. C. Hong, “Efficient and quantitative chemical transformation of vegetable oils to polyols through a thiol-ene reaction for thermoplastic polyurethanes,” Ind. Crops Prod., vol. 87, pp. 78–88, 2016. |
| [13] | P. Alagi, Y. J. Choi, and S. C. Hong, “Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane,” Eur. Polym. J., vol. 78, pp. 46–60, 2016. |
| [14] | E. Sharmin, M. T. Kafyah, A. A. Alzaydi et al., “Synthesis and characterization of polyvinyl alcohol/corn starch/linseed polyol-based hydrogel loaded with biosynthesized silver nanoparticles,” Int. J. Biol. Macromol., vol. 163, pp. 2236–2247, Nov. 2020. |
| [15] | Z. Dai, P. Jiang, W. Lou et al., “Preparation of degradable vegetable oil-based waterborne polyurethane with tunable mechanical and thermal properties,” Eur. Polym. J., vol. 139, no. September, p. 109994, 2020. |
| [16] | D. Miloslavskiy, E. Gotlib, О. Figovsky, and D. Pashin, “Cyclic Carbonates Based on Vegetable Oils,” Int. Lett. Chem. Phys. Astron., vol. 27, pp. 20–29, 2014. |
| [17] | S. Doley and S. K. Dolui, “Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties,” Eur. Polym. J., vol. 102, pp. 161–168, 2018. |
| [18] | B. Tamami, S. Sohn, and G. L. Wilkes, “Incorporation of Carbon Dioxide into Soybean Oil and Subsequent Preparation and Studies of Nonisocyanate Polyurethane Networks,” J. Appl. Polym. Sci., vol. 92, pp. 883–891, 2004. |
| [19] | I. Javni, D. P. Hong, and Z. S. Petrović, “Soy-based polyurethanes by nonisocyanate route,” J. Appl. Polym. Sci., vol. 108, no. 6, pp. 3867–3875, Jun. 2008. |
| [20] | A. R. Mahendran, N. Aust, G. Wuzella, U. Müller, and A. Kandelbauer, “Bio-Based Non-Isocyanate Urethane Derived from Plant Oil,” J. Polym. Environ., vol. 20, no. 4, pp. 926–931, 2012. |
| [21] | M. A. Sawpan, “Polyurethanes from vegetable oils and applications: a review,” J. Polym. Res., vol. 25, no. 8, 2018. |
| [22] | A. R. Mahendran, G. Wuzella, N. Aust, and U. Müller, “Synthesis, characterization, and properties of isocyanate-free urethane coatings from renewable resources,” J. Coatings Technol. Res., vol. 11, no. 3, pp. 329–339, 2014. |
| [23] | M. Bähr and R. Mülhaupt, “Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion,” Green Chem., vol. 14, no. 2, pp. 483–489, 2012. |
| [24] | S. Miao, P. Wang, Z. Su, and S. Zhang, “Vegetable-oil-based polymers as future polymeric biomaterials,” Acta Biomater., vol. 10, no. 4, pp. 1692–1704, 2014. |
| [25] | S. J. Park, F. L. Jin, and J. R. Lee, “Synthesis and thermal properties of epoxidized vegetable oil,” Macromol. Rapid Commun., vol. 25, no. 6, pp. 724–727, 2004. |
| [26] | A. R. Mahendran, N. Aust, G. Wuzella, and A. Kandelbauer, “Synthesis and characterization of a bio-based resin from linseed oil,” Macromol. Symp., vol. 311, no. 1, pp. 18–27, 2012. |
| [27] | S. G. Tan and W. S. Chow, “Biobased epoxidized vegetable oils and its greener epoxy blends: A review,” Polym. - Plast. Technol. Eng., vol. 49, no. 15, pp. 1581–1590, 2010. |
| [28] | T. Saurabh, M. Patnaik, S. L. Bhagt, and V. C. Renge, “Epoxidation of vegetable oils: a review,” Int. J. Adv. Eng. Technol, vol. 2, no. Iv, pp. 491–501, 2011. |
| [29] | B. M. Abdullah and J. Salimon, “Epoxidation of vegetable oils and fatty acids: Catalysts, methods and advantages,” Journal of Applied Sciences, vol. 10, no. 15. pp. 1545–1553, 2010. |
| [30] | E. Dehonor Márquez, J. Nieto Alarcón, E. Vigueras Santiago, and S. Hernández López, “Effective and Fast Epoxidation Reaction of Linseed Oil Using 50 wt% Hydrogen Peroxyde,” Am. J. Chem., vol. 8, no. 5, pp. 99–106, 2018. |
| [31] | S. Dinda, A. V. Patwardhan, V. V. Goud, and N. C. Pradhan, “Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids,” Bioresour. Technol., vol. 99, no. 9, pp. 3737–3744, 2008. |
| [32] | V. V. Goud, A. V. Patwardhan, and N. C. Pradhan, “Studies on the epoxidation of mahua oil (Madhumica indica) by hydrogen peroxide,” Bioresour. Technol., vol. 97, no. 12, pp. 1365–1371, 2006. |
| [33] | D. C. Webster, “Cyclic carbonate functional polymers and their applications,” Prog. Org. Coatings, vol. 47, no. 1, pp. 77–86, 2003. |
| [34] | M. Musik and E. Milchert, “Selective epoxidation of sesame oil with peracetic acid,” Mol. Catal., vol. 433, pp. 170–174, 2017. |
| [35] | C. Cai, H. Dai, R. Chen et al., “Studies on the kinetics of in situ epoxidation of vegetable oils,” Eur. J. Lipid Sci. Technol., vol. 110, no. 4, pp. 341–346, Apr. 2008. |
| [36] | M. Jebrane, S. Cai, C. Sandström, and N. Terziev, “The reactivity of linseed and soybean oil with different epoxidation degree towards vinyl acetate and impact of the resulting copolymer on the wood durability,” Express Polym. Lett., vol. 11, no. 5, pp. 383–395, 2017. |
| [37] | M. R. Janković, O. M. Govedarica, and S. V. Sinadinović-Fišer, “The epoxidation of linseed oil with in situ formed peracetic acid: A model with included influence of the oil fatty acid composition,” Ind. Crops Prod., vol. 143, no. June 2019, 2020. |
| [38] | A. F. Aguilera, P. Tolvanen, S. Heredia et al., “Epoxidation of Fatty Acids and Vegetable Oils Assisted by Microwaves Catalyzed by a Cation Exchange Resin,” Ind. Eng. Chem. Res., vol. 57, no. 11, pp. 3876–3886, 2018. |
| [39] | A. B. Kousaalya, S. D. Beyene, V. Gopal, B. Ayalew, and S. Pilla, “Green epoxy synthesized from Perilla frutescens: A study on epoxidation and oxirane cleavage kinetics of high-linolenic oil,” Ind. Crops Prod., vol. 123, no. June, pp. 25–34, 2018. |
| [40] | M. Kurańska, H. Beneš, A. Prociak, O. Trhlíková, Z. Walterová, and W. Stochlińska, “Investigation of epoxidation of used cooking oils with homogeneous and heterogeneous catalysts,” J. Clean. Prod., vol. 236, p. 117615, Nov. 2019. |
| [41] | S. K. Sahoo, V. Khandelwal, and G. Manik, “Development of toughened bio-based epoxy with epoxidized linseed oil as reactive diluent and cured with bio-renewable crosslinker,” Polym. Adv. Technol., vol. 29, no. 1, pp. 565–574, 2018. |
| [42] | S. K. Sahoo, V. Khandelwal, and G. Manik, “Synthesis and characterization of low viscous and highly acrylated epoxidized methyl ester based green adhesives derived from linseed oil,” Int. J. Adhes. Adhes., vol. 89, no. January, pp. 174–177, Mar. 2019. |
| [43] | J. F. Nieto Alarcón, “Estudio de la reacción de carbonatación del aceite de linaza epoxidado,” Tesis de Maestría en Ciencia de Materiales. Facultad de Química. Universidad Autónoma del Estado de México, 2019. |
| [44] | B. P. S. B. Dobbinsin, W. Hofmann, The Determination of Epoxide Groups, First. Pergamon Press, 1969. |
| [45] | F. Gustone, Rapessed and canola oil: production, processing, properties and uses. Oxford: Blackwell Publishing, 2004. |
| [46] | Y. M. Evtushenko, V. M. Ivanov, and B. E. Zaitsev, “Determination of Epoxide and Hydroxyl Groups in Epoxide Resins by IR Spectrometry,” J. Anal. Chem., vol. 58, no. 4, pp. 347–350, 2003. |
| [47] | G. L. Téllez, E. Vigueras-Santiago, and S. Hernández-López, “Characterization of linseed oil epoxidized at different percentages,” Superf. y Vacío, vol. 22, no. 1, pp. 5–10, 2009. |
| [48] | M. Farias, M. Martinelli, and D. P. Bottega, “Epoxidation of soybean oil using a homogeneous catalytic system based on a molybdenum (VI) complex,” Appl. Catal. A Gen., vol. 384, no. 1–2, pp. 213–219, 2010. |
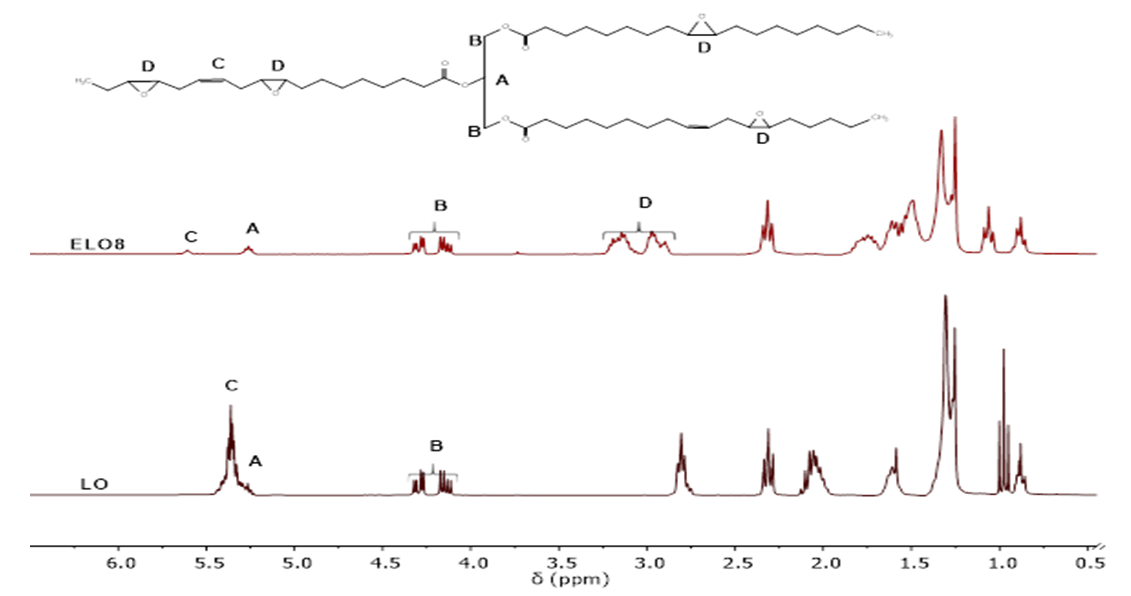
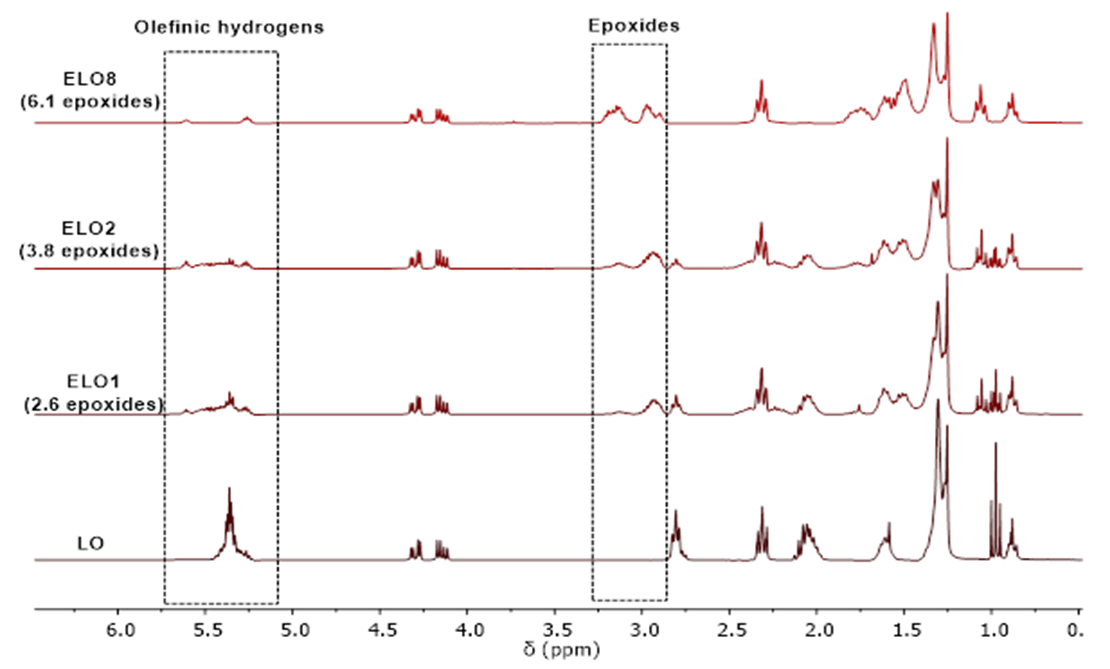
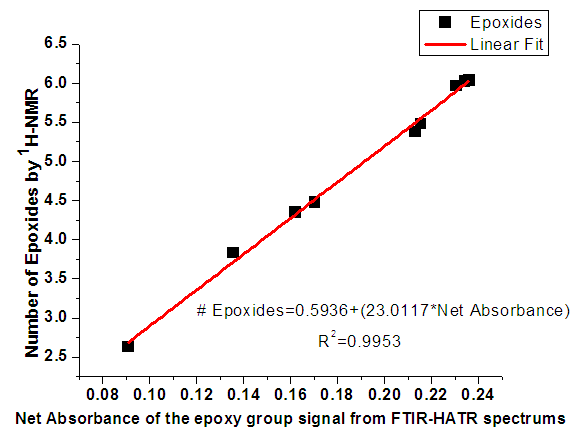
| [49] | W. Xia, S. M. Budge, and M. D. Lumsden, “1H-NMR Characterization of Epoxides Derived from Polyunsaturated Fatty Acids,” JAOCS, J. Am. Oil Chem. Soc., vol. 93, no. 4, pp. 467–478, 2016. |
| [50] | R. T. Alarcon, C. Gaglieri, K. J. Lamb, M. North, and G. Bannach, “Spectroscopic characterization and thermal behavior of baru nut and macaw palm vegetable oils and their epoxidized derivatives,” Ind. Crops Prod., vol. 154, p. 112585, Oct. 2020. |
| [51] | Y. Awoke, Y. Chebude, C. Márquez-Álvarez, and I. Díaz, “Solvent free epoxidation of vernonia oil using Ti-SBA-15 with tailor made particle morphology and pore size,” Catal. Today, vol. 345, no. September, pp. 190–200, Apr. 2019. |
| [52] | S. T. Anuar, Y. Y. Zhao, S. M. Mugo, and J. M. Curtis, “Monitoring the epoxidation of canola oil by non-aqueous reversed phase liquid chromatography/mass spectrometry for process optimization and control,” JAOCS, J. Am. Oil Chem. Soc., vol. 89, no. 11, pp. 1951–1960, 2012. |
| [53] | M. González-González, J. C. Cabanelas, and J. Baselga, “Applications of FTIR on Epoxy Resins - Identification, Monitoring the Curing Process, Phase Separation and Water Uptake,” Infrared Spectrosc. - Mater. Sci. Eng. Techology, vol. 2, pp. 261–284, 2012. |
| [54] | C. Núñez, E. Vergara, J. Lisperguer, and C. Droguett, “Fast and reliable method for monitoring epoxidation reaction in recycled vegetable oils (ERVO) using FTIR-ATR technique,” J. Chil. Chem. Soc., vol. 61, no. 1, pp. 2763–2766, 2016. |
| [55] | Z. A. Bakar, Z. Idris, A. Hazmi et al., “Process control for epoxidation of rbd palm olein,” J. Oil Palm Res., vol. 28, no. 2, pp. 228–233, 2016. |
| [56] | P. D. Meshram, R. G. Puri, and H. V. Patil, “Epoxidation of wild safflower (Carthamus oxyacantha) oil with peroxy acid in presence of strongly acidic cation exchange resin IR-122 as catalyst,” Int. J. ChemTech Res., vol. 3, no. 3, pp. 1152–1163, 2011. |
| [57] | P. Joseph-Nathan and E. Díaz T., Introducción a la Resonancia Magnética Nuclear, Primera Re. México: Editorial Limusa, S.A., 1980. |
| [58] | X. Zhao, T. Zhang, Y. Zhou, and D. Liu, “Preparation of peracetic acid from hydrogen peroxide. Part I: Kinetics for peracetic acid synthesis and hydrolysis,” J. Mol. Catal. A Chem., vol. 271, no. 1–2, pp. 246–252, 2007. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML