-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2020; 10(2): 20-25
doi:10.5923/j.aac.20201002.02
Received: Nov. 3, 2020; Accepted: Nov. 30, 2020; Published: Dec. 15, 2020

Antioxidant Activity of Five Medicinal Plants Extrats Used for Management of Bacterial Diseases in Burkina Faso
Jules Yoda1, 2, Seydou Sourabié1, 3, Angèle Zoungrana1, 3
1Department of Medicine, Traditional Pharmacopeia and Pharmacy (MEPHATRA/Ph)/ Research Institute for Health Sciences (IRSS), Ouagadougou, Burkina Faso
2Laboratory of Molecular Chemistry and Materials (LC2M), Team of Organic Chemistry and Phytochemistry (ECOP), University Joseph Ki-Zerbo, Ouagadougou, Burkina Faso
3Pharmacognosy Laboratory, (UFR/SDS), University Joseph KI-ZERBO, Ouagadougou, Burkina Faso
Correspondence to: Jules Yoda, Department of Medicine, Traditional Pharmacopeia and Pharmacy (MEPHATRA/Ph)/ Research Institute for Health Sciences (IRSS), Ouagadougou, Burkina Faso.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The plant biodiversity of Burkina Faso abounds with countless plants, some of which are of proven medicinal interest. The objective of this work is to highlight five (5) medicinal plants including Psidium guajava, Euphorbia hirta, Combretum micranthum, Eucalyptus camaldulensis and Terminalia avicennioides used in the management of bacterial infections. Recent work shows that many microbial pathologies, particularly bacterial and viral infections, are linked to oxidative stress induced by the production and development of free radicals. In this study, we established the chemical profile of the extracts in therapeutic compounds of interest such as phenolic compounds. The antioxidant activity of the decocted extracts of these five plants was studied by several methods including the DPPH tests, the ABTS test and the lipid peroxidation test. Significant and interesting results were obtained.
Keywords: Traditional medicine, Antibacterial activity, Phytochemical screening, Antioxidant, Activity
Cite this paper: Jules Yoda, Seydou Sourabié, Angèle Zoungrana, Antioxidant Activity of Five Medicinal Plants Extrats Used for Management of Bacterial Diseases in Burkina Faso, Advances in Analytical Chemistry, Vol. 10 No. 2, 2020, pp. 20-25. doi: 10.5923/j.aac.20201002.02.
Article Outline
1. Introduction
- The discovery of antibiotics was a real revolution in the fight against infectious diseases. However, inappropriate consumption and misuse of antibiotics has accelerated the selection of multi-resistant bacteria, which is currently a real problem for antibiotic therapy and public health [1]. Thus, the use of natural resources in general and medicinal plants in particular is emerging as one of the most important and interesting approaches to explore in the search for new and more effective antibacterial drugs [2]. In addition, it is recognized in the scientific community that products of natural origin are an important source of therapeutic agents for microbial diseases that claim a large number of victims in terms of mortality rates [3-5]. Indeed, antimicrobial compounds extracted from certain plants can inhibit bacterial growth by different mechanisms. The interest of our work lies in the study of the antioxidant activity of five plants including Psidium guajava L., Euphorbia hirta L., Combretum micranthum G. Don, Eucalyptus camaldulensis Dehn and Terminalia avicennioides Guill. & Perr.. The antibacterial activity of these plants is well known, and their role in the fight against oxidative stress induced during infections is being investigated. In the defense process of the organism during infections, there is the production of free radicals by various mechanisms. The anti-radical activity of a compound is associated with its ability to prevent or delay oxidation for as long as possible [6]. This oxidation can be the result of free radicals whose production is increased during the development of many pathologies such as obesity, diabetes, atherosclerosis, cancer, bacterial and viral diseases [7-10]. Evaluation of phytotherapeutic properties, such as antibacterial and antioxidant activity is considered very important and useful, especially for plants, which are widely used in traditional medicine. Therefore, the present study aims to highlight the antioxidant capacity of the extracts of these five plants with antibacterial properties used in the treatment of bacterial infections in traditional medicine in Burkina Faso. We performed a chemical screening of the extracts from the decoction in order to characterize the main chemical compounds, particularly phenolic compounds. The analysis of the antioxidant properties of the different extracts uses three different methods.
2. Materials and Methods
2.1. Plant Material and Moisture Content
- The plant material was identified by Dr. Souleymane GANABA, in charge of the Ecosystem Management and Monitoring Program of the Environment and Forest Department of the Institute of Environment and Agricultural Research (INERA-Burkina Faso). The plant material consisted of organs of the five (5) species including the leaves of Psidium guajava, the leaves of Combretum micranthum, the leaves of Eucalyptus camaldulensis, the whole plant of Euphorbia hirta and the leaves of Terminalia avicennioides. These plant parts were collected during month of March 2019 in two locations. The moisture content of the powders was obtained according to the thermogravimetric method [11].
 | Figure 1. Psidium guajava: (A) whole plant and (B) leaves |
2.2. Extraction
- The preparation of the extracts of the study consisted in carrying out an aqueous and hydro-ethanolic decoction of the various powders of the five (5) plants with 50 g of each powder in 500ml of solvent. The mixture was homogenized and boiled for 30 minutes. After cooling, the extracts were filtered and centrifuged at 2000 rpm for 10 minutes and dry concentrated using a freeze dryer. In the case of hydroalcoholic extracts, the mixture was first concentrated on a rotavaporizer to remove the organic solvent.
2.3. Determination of the Phytochemical Constituent
- The phytochemical screening was carried out according to the methods already described in the literature [12-13]. All tests were performed using chromatoplaques (60 F250, glass support 20 x 20, Fluka-Silica gel). The chemical characterization tests on chromatoplate were performed by vaporization of the different spots obtained after a thin layer separation of the constituents of the extracts with general and specific reagents: FeCl3 reagent for tannins and phenolic compounds, sulfuric vanillin reagent for sterols and terpenes, Neu reagent for flavonoids, 5% (V/V) methanolic KOH reagent for coumarins and sulfuric anisaldehyde reagent for saponosides.. The chromatographic profile of the extract samples was observed in daylight and under ultraviolet radiation (254 nm and 366 nm) before and after spraying with the different reagents.
2.4. Determination of Total Phenols
- Quantitative analysis of compounds of interest concerns the most cited plant metabolites in favour of antimicrobial fight. We have therefore determined the content of phenolic compounds in general. The reference compounds used is gallic acid. The determination of the amount of total polyphenols in plant extracts uses a classical method that takes into account the Folin-Ciocalteu reagent [11]. Folin-Ciocalteu is a mixture of phosphotungstic acid and phosphomolybdic acid which is reduced during the oxidation of phenols in an alkaline medium to a mixture of blue oxides of tungsten and molybdenum. The latter have an absorption maximum at 760 nm whose intensity is proportional to the amount of polyphenols present in the sample.
2.5. Antioxidant Activity
2.5.1. ABTS Radical Reduction Test
- This test is based on the oxidation-reduction mechanism of ABTS (ammonium salt of 2,2'- azino bis-(3-ethylbenzothiazoline-6-sulfonic acid). During this test the ABTS salt loses an electron to form a dark-coloured cation radical in solution. In the presence of the antioxidant agent, the radical formed is reduced to give the ABTS cation causing the solution to discolour. The method used is that described by Arts et al [14]. Briefly, the primary concentration of the samples (5mg/ml), a series of eight (08) successive dilutions was performed on a 96-well microplate. Trolox was used as reference substance. The whole was protected from light for 30 minutes at 25°C and the absorbances were read at 734 nm with the spectrophotometer.
2.5.2. DPPH Radical Reduction Test
- Method for measuring the antioxidant capacity by DPPH (2,2-Diphenyl-picrylhydrazine) method is based on the ability of a compound to reduce the DPPH radical. The reduction results in a change in colour of the solution from purple to yellow in the presence of an anti-radical compound. The reaction is then quantified by measuring the absorbance of the solution by spectrophotometry at 490 nm. This test used to determine the anti-radical activity of extracts is based on the methodology developed by Takao and modified by Sombie [15]. Thus, a series of concentrations were performed from primary concentration (1mg/ml) of the samples and the trolox. On a 96-well microplate, the filling of each well for each concentration was done and deposited on a plate with 200 μL of DPPH solution (0.04mg/ml) and 20 μL of the diluted extract or reference. After 30 minutes of incubation, the absorbance was read at the wavelength of 490 nm using a spectrophotometer. The blank without sample was prepared under the same conditions and consisted of 200 μL DPPH and 20 μL ethanol. A curve of the percentage inhibition of DPPH was plotted as a function of sample concentration. On the curve, the concentration required to degrade 50% DPPH (IC50) was determined.
2.5.3. Inhibition of Lipid Peroxidation
- The evaluation of lipid peroxidation was carried out by determining the substances reacting with thiobarbituric acid. The analysis is based on the formation of a pink complex in an acidic and hot environment that absorbs at 532 nm [16]. In this method 40 μL extracts (10 mg/mL) were mixed at 200μL liver homogenate in 1% Tris-HCl buffer (50Mm, pH 7.40), then 10 μL FeCl2 (0.5 mM) and 10 μL H2O2 (0.5 mM) were added. The mixture was incubated at 37°C for 60 minutes, then 200μL trichloroacetic acid (TCA) (15%) and 200μL 2-thiobarbituric acid (0.67%) were added and the mixture was heated in boiling water (100°C) for 15 minutes. Absorbances were read at 532 nm using the spectrophotometer. Trolox was used as reference compound. The ability of the extracts to inhibit lipid peroxidation of the liver is expressed as a percentage inhibition.
3. Results and Discussion
3.1. Phytochemical Analysis
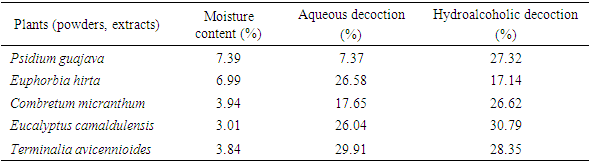
- Moisture content in the samples ranged from 3.84% ± 0.11 to 7.39% ± 0.08 (Table 1). High a percentage of water in a drug promotes a number of enzymatic reactions that can have negative consequences on the appearance of the drugs, their organoleptic characteristics, their therapeutic properties by degradation of the active compounds over time [11]. Thus, the content allowed in a drug for its good conservation should not exceed 10% [17]. Therefore, the moisture content of all our powders being less than 10%, suggests that our plant drugs could be preserved for a long period of time with less risk of contamination and/or alteration of the chemical principles. The different powders were extracted by aqueous and hydroalcoholic decoction. The extraction yields are reported in the table below (Table 1). In the case of the two extraction methods, the aqueous decoction gives the best yield with Euphorbia hirta and Terminalia avicennioides. The three other samples give quantitative extracts (yields > 17%) in the case of the hydroalcoholic decoction.
|
3.2. Phytochemical Screening
- Phytochemical screening by thin layer chromatography (TLC) was aimed to determine the different chemical groups involved in the antibacterial activity for the different extracts (a and b). The main research groups are listed in the table below (Table 2).
|
3.3. Total Phenols Content
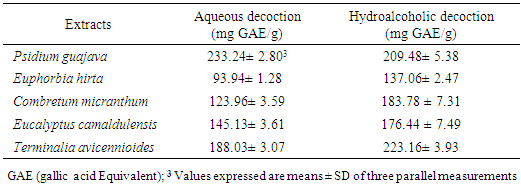
- Dosage of phenolic compounds is a common practice in the evaluation of the therapeutic properties of medicinal plants. Thus, the choice to quantify them among the different phytochemicals would result from the fact that these compounds possess interesting biological activities due to their presence. The content of total phenolic compounds was evaluated by the method of Folin-Cioalteu and for this purpose, we established a calibration curve from the gallic acid used as reference. The extrapolation of the content of total phenolic compounds contained in the fractions was done using the gallic acid curve equation. The results obtained are expressed in milligram Gallic Acid equivalent /100g extract (mgGAE/100g) and are shown in the table and figure below (Table 3; figure 2). The highest content was obtained with Psidium guajava (233.24 ± 2.803mg GAE/g) for the aqueous extracts. For the hydroalcoholic extracts, the contents are high for all the extracts (> 130 mg GAE/g). the highest contents for these extracts are obtained particularly with Psidium guajava (209.48 ± 5.38 mg GAE/g) and Terminalia avicennioides (223.16 ± 3.93 mg GAE/g). These levels show that our plants are rich in phenolic compounds. These results are in line with studies already realized on the antioxidant properties of Psidium guajava leaves which would be linked to the high content of phenolic compounds in the tested extracts. These properties could justify the beneficial effects of diabetisane based on Psidium guajava leaves in the prevention of diabetes and its degenerative complications. In addition, polyphenols are known for their antioxidant capacity [25].
|
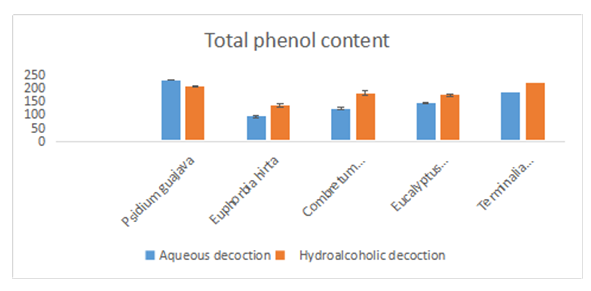 | Figure 2. Dosage of total phenolic compounds |
3.4. Antioxidant Activity
3.4.1. Determination of Antiradical Activity
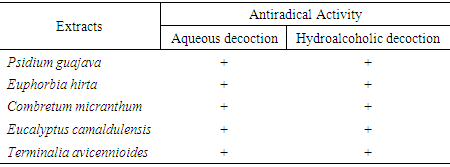
- The positive (+) test is indicated on the plate as a luminous area on a purple background (figure 3). This explains the reaction between the DPPH reagent and the compounds present in the extracts. This reaction shows that the extracts contain phytocompounds that can trap free radicals [26]. All the extracts submitted to this test gave a positive result, which explains the antioxidant properties of the different extracts. By superimposing the chromatographic profiles of the phytochemical screening and those of the antiradical activity, it is possible to establish the correspondence between the active zones and the identified phytocompounds.
|
 | Figure 3. Anti-radical test using TLC plate |
3.4.2. ABTS and DPPH Methods
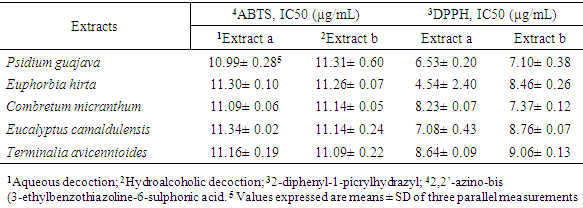
- The evaluation of the antioxidant activity by the ABTS and DPPH methods was determined from the different IC50s, i.e. the concentration of the extract/fraction likely to cause a 50% inhibition. The results are presented in the table below (Table 5).
|
3.4.3. Inhibition of Lipid Peroxidation
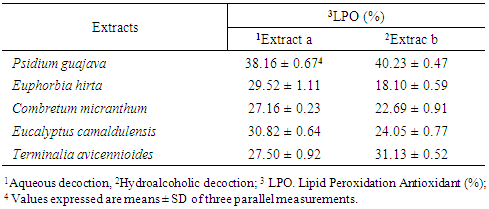
- The results of the inhibition of lipid peroxidation of the liver are presented in the table below (Table 6).
|
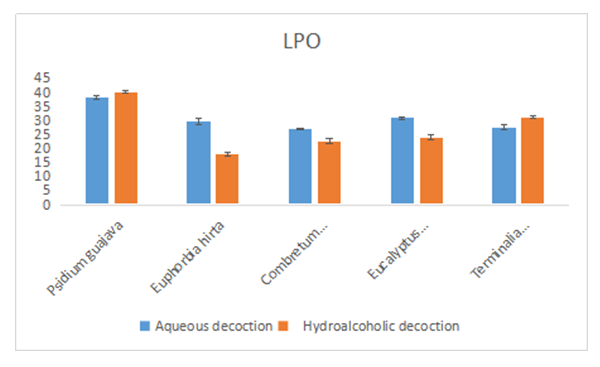 | Figure 4. Results of the inhibition test of lipid peroxidation |
4. Conclusions
- This study allowed us to qualitatively analyze the presence of compounds of interest such as phenolic compounds. The extracts each present an antioxidant activity with varying antioxidant capacity Among the five plants, Psidium guajava extracts have the highest content of total phenolic compounds and also show the highest antioxidant activity according to different tests. For a possible association of these plants, it would be more beneficial to take into account the presence of psiduim guajava. As a perspective, we plan to study some associations, especially those already made by the populations with an inclusion of psidium guajava. Since the chemical profiles are already known, we will test the antioxidant power and antibacterial properties of the combinations that will be developed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML