-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2020; 10(2): 15-19
doi:10.5923/j.aac.20201002.01
Received: Oct. 15, 2020; Accepted: Nov. 5, 2020; Published: Nov. 15, 2020

Evaluation of Warburgia ugandensis Extracts and Compounds for Crop Protection against Prostephanus truncates
Sylvia A. Opiyo
Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya
Correspondence to: Sylvia A. Opiyo, Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Insect pests cause a significant loss of maize production in Africa. Maize weevil (Sitophilus zeamais Motchulsky) and the larger grain borer (Prostephanus truncatus Horn) are the most destructive insect pests of maize. Chemical control is the most commonly used and the most effective method at the farm level. However, some of the chemicals cause adverse effects to environment and humans. In addition, insecticides available in the market are expensive and mostly out of reach to smallholder farmers. The use of botanicals for pests and disease control is preferred because plant materials are non-toxic and are readily available. This study investigated the efficacy of extracts and compounds from Warbugia ugandensis leaves for control of P. truncatus infestation in stored maize. All the crude extracts exhibited repellent, toxicity and growth inhibition activities against P. truncates. The most active compounds were polygodial, warburganal, ugandensolide and mukaadial. The findings from this study show that extracts from W. ugandensis are effective in controlling the larger grain borer and therefore could be used to control the pest.
Keywords: Warbugia ugandensis, Insect pests, Prostephanus truncates, Repellence, Mortality, Growth inhibition
Cite this paper: Sylvia A. Opiyo, Evaluation of Warburgia ugandensis Extracts and Compounds for Crop Protection against Prostephanus truncates, Advances in Analytical Chemistry, Vol. 10 No. 2, 2020, pp. 15-19. doi: 10.5923/j.aac.20201002.01.
Article Outline
1. Introduction
- Insect pests cause significant losses of maize in Africa, reducing the 4.9 t/ha world average grain yield production to 1.5 t/ha average [1,2]. Infestation by post-harvest pests commences in the field but most damage occurs during storage [3]. Among pests of maize, beetles are the most important, with maize weevil (Sitophilus zeamais Motchulsky) and larger grain borer (Prostephanus truncatus Horn) being the major ones. The extensive tunneling in maize grain by pests allows the pests to convert maize grain into flour within a very short time [3]. Small-scale farmers are often forced to sell maize shortly after harvest to minimize losses during storage, thereby attract low prices and compromising food security at the house hold level. Technologies that can reduce yield losses from storage pest damage are necessary to increase maize production to cope with increasing demand for maize in Kenya. Chemical control method is the most commonly used and most effective at the farm level. Pesticides such as organochlorines, organophosphates, carbamates, organoarsenicals and organothiocynates have been recommended to control the weevils [4]. However, some of the chemicals have adverse effects on environment and humans [5]. Banning some of the chemicals has lest just a few insecticidal alternatives for pest control operations. Furthermore, insecticides are expensive and mostly out of reach to smallholder farmers [6]. There is urgent need to develop safe alternatives that are of readily available, convenient to use and environmentally friendly [7].Plant extracts contain secondary metabolites some of which inhibit the growth of pests and pathogenic microorganism [8-13]. The use of botanical for pests and disease control is preferred because they are safe and non-toxic to humans [14-19]. In addition, chances of pests and pathogens developing resistance to botanical pesticides are highly unlikely [20]. Warbugia ugandensis (Canellaceae) is traditionally used as a remedy for stomachache, constipation, toothache, malaria, sexually transmitted diseases, diarrhoea, cough and internal wounds/ulcers [21]. Warburgia species are characterized by the presence of drimane sesquiterpenes some of which have been reported to exhibit antibacterial, antifungal, insect antifeedant, insecticidal and molluscicidal activities [8,22,23]. The present study was conducted to investigate the efficacy of extracts and compounds of W. ugandensis in controlling of P. truncatus infestation in stored maize.
2. Materials and Methods
2.1. Plant Materials
- Leaves of W. ugandensis were collected along Nakuru Gilgil Highway near St. Mary's Hospital (latitude 0° 24' 42.49'' S and longitude 36° 15' 10.59'' E) in May 2014 and voucher specimen (2014/5/SAO/CHEMMK) was identified at the Kenya National Museum Herbarium after comparison with authentic samples. The plant materials were air dried at 24-28°C until crispy. The dried leaves were pulverized and sieved through a 0.5 mm size mesh.
2.2. Extraction and Isolation of Compounds
- Two kg of powdered leaves of W. ugandensis was cold extracted with organic solvents of varying polarities (n-hexane, ethyl acetate and methanol) sequentially by soaking in the solvents for seven days with occasional shaking. The mixture was filtered and concentrated using a rotary evaporator at reduced pressure to yield 20.2, 58.6 and 97.8 g of n-hexane, ethyl acetate and methanol extracts, respectively. The resultant extracts were stored at 4°C for bioassays and phytochemical studies. n-Hexane and ethyl acetate extracts showed similar TLC profile and were combined for phytochemical isolation. The combined extract (50 g) was dissolved in a small amount of n-hexane – ethyl acetate mixture (1:1) and subjected to in silica gel for column chromatography using silica gel. Elution was done using n-hexane, n-hexane - ethyl acetate mixture, ethyl acetate and methanol to give 200 fractions (each 20 ml) whose compositions were monitored by TLC and those with similar profiles were combined to give seven pools labeled I -VII. Pool I, 3g, which was eluted with n-hexane did not show any major spot on TLC and was discarded. Pool II (7 g) was subjected to further column chromatography eluting with n-hexane: ethyl acetate (95:5, 9:1, 85:15 and 4:1) to give polygodial (1) 30 mg and warbuganal (2) 55 mg. Pool IV (5 g) on further fractionation with gradient n-hexane-ethyl acetate mixture (85:15, 4:1 and 7:3) gave polygodial (1) 35 mg and ugandensolide (3) 38 mg. Pool V (8 g) on further fractionation with n-hexane: ethyl acetate (4:1, 7:3 and 65:35) gave ugandensolide (3) 24 mg, ugandensidial (4) 42 mg and muzigadial (5) 75 mg. Pool VI (9 g) gave ugandensidial (4) 43 mg while Pool VII gave muzigadial (5) 15 mg and mukaadial (6) 72 mg.
2.3. Mass Rearing of Prostephanus truncatus
- Adult weevils were obtained from infested maize grains purchased from a local market and from the stock, new generation was reared on dry pest susceptible maize grains [24]. Two hundred maize weevils of mixed sexes were introduced into a two liter glass jars containing 400 g weevil susceptible maize grains [25]. The mouths of the jars were then covered with nylon mesh held in place with rubber bands and the jars left undisturbed for 35 days for oviposition. Thereafter, all adults were removed through sieving and each jar was left undisturbed for another 35 days. Emerging adult insects were collected and kept in separate jars according to their age. Adults that emerged on same day were considered of the same age [26].
2.4. Repellency Test
- The test was done according to [24] with some modifications. Transparent plastic tubings, 13 cm long x 1.3 cm diameter were used as test cylinders. Each test cylinder was plugged at one end with cotton ball containing solid crude extracts and compound isolated from the stem bark of W. ugandensis while the other end was plugged with clean cotton ball which served as control. Actellic dust was used as a positive control. Ten-three-day old unsexed test insects were introduced at the middle of each test cylinder through a hole at the middle portion of the cylinder (0.0 cm) and let to move in any direction of their choice with scoring of distance moved measured in cm using a ruler. The score time was 24 hours after exposure and all tests were done in triplicates.
2.5. Adult Mortality Test
- Contact toxicity assay was done according to Ileke and Oni [27] with some modifications. Toxicity of the crude extracts and isolated compounds were tested against adult weevils. The test samples were mixed with talc thoroughly and the dust was admixed with 20 g of maize held in 12 cm high x 6.5 cm diameter glass jars covered with ventilated lids. To ensure a thorough admixture, the grain was put in 12 cm high x 6.5 cm diameter glass jars, dust applied and top lid replaced. The grain was then swirled within the jar until a proper admixture was realized [28]. Twenty-three-day old unsexed insect pairs were then introduced into each dish and exposed to treatments. Actellic dust was used as a positive control and all tests were done in three replicates. Maize weevils were considered dead when probed with sharp objects and there were no responses [27]. The number of dead insects in each vial was counted after 21 days after treatment to estimate maize weevil mortality as follows:
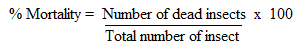 Data on percentage adult weevil mortality were corrected using Abbott’s formula [29]: PT = (Po – Pc) / (100 - Pc)Where PT = Corrected mortality (%); Po = Observed mortality (%); PC = Control mortality (%).
Data on percentage adult weevil mortality were corrected using Abbott’s formula [29]: PT = (Po – Pc) / (100 - Pc)Where PT = Corrected mortality (%); Po = Observed mortality (%); PC = Control mortality (%).2.6. Growth Inhibition Assay
- The test was done according to Ileke and Oni [27] with some modifications. 20 g of clean undamaged and uninfected corn grains were placed in 12 cm high x 6.5 cm diameter glass jars glass jars. Test materials in powder form were thoroughly mixed with the grains in each jar. Crude extracts and pure compounds were mixed with talc thoroughly before being applied to the grains [28]. A mixture of twenty-seven-day old unsexed maize weevils was introduced in each jar and covered with filter paper [26]. The female adults were allowed to oviposit on the seeds for 4 days. On day 5, all insects were removed from each container and the seeds returned to their respective containers. Progeny emergence (F1) was recorded at six weeks (42 days). The containers were sieved out and newly emerged adult weevils were counted [27]. At week six, the grains were reweighed and the percentage loss in weight was determined as follow:
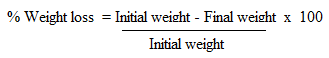
3. Results and Discussion
3.1. Phytochemical Studies
- Fractionation of n-hexane and ethyl acetate extracts from W. ugandensis stem bark over silica gel column afforded six compounds (Figure 1) namely polygodial (1), warbuganal (2), ugandensolide (3), ugandensidial (4), muzigadial (5) and mukaadial (6) [22,30].
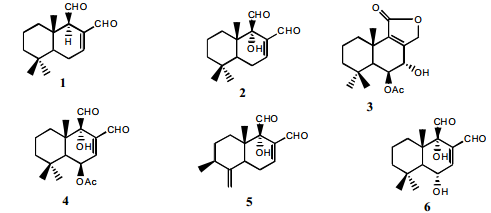 | Figure 1. Structures of the tested compounds from W. ugangensis [22] |
3.2. Repellent, Toxicity and Growth Inhibition Activities
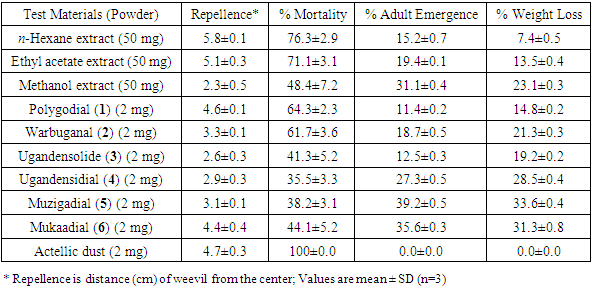
- Crude extracts (n-hexane, ethyl acetate and methanol) and compounds (polygodial (1), warbuganal (2), ugandensolide (3), ugandensidial (4), muzigadial (5) and mukaadial (6) isolated from the leaves of W. ugandensis were testes for repellence, toxicity and growth inhibition activities against the larger grain borer (Table 1). n-Hexane and ethyl acetate extracts (mean repellency = 5.8 & 5.1 cm, respectively) repelled the insects more than Actellic dust (mean repellency = 4.7 cm) which was used as a positive control. The repellence activity exhibited by the isolated compounds polygodial (1) and mukaadial (6) (mean repellency = 4.6 & 4.4 cm, respectively) were comparable to that of the standard.
|
4. Conclusions
- The insect repellence, mortality and growth inhibition activities tests with W. ugandensis have demonstrated that n-hexane and ethyl extract are effective in controlling larger grain borer which is one of the most important insects pets of maize grains. Among the isolated compounds, polygodial (1), warburganal (2), ugandensolide (3) and mukaadial (6) were the most potent for control of the insect.
ACKNOWLEDGEMENTS
- The authors are grateful to the National Commission for Science, Technology and Innovation (NACOSTI) and Biosciences Eastern and Central Africa Network (BecANet) for financial support. We also acknowledge the assistance from Chemistry Department, Maseno University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML