-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2020; 10(1): 7-10
doi:10.5923/j.aac.20201001.02

Application of Different Ligands to Optimize Cd(II) Removal Through Liquid Membranes
Mersiha Suljkanović1, Edita Bjelić2, Jasmin Suljagić2, Azra Kovačević2
1Faculty of Natural Sciences and Mathematics, University of Tuzla, Tuzla, Bosnia and Herzegovina
2Faculty of Technology, University of Tuzla, Tuzla, Bosnia and Herzegovina
Correspondence to: Mersiha Suljkanović, Faculty of Natural Sciences and Mathematics, University of Tuzla, Tuzla, Bosnia and Herzegovina.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Among numerous techniques for successful separation and removal of metal cations, transport through bulk liquid membranes attracts special attention of researchers. In this paper, different ligands were applied as possible carriers for Cd(II) ions. Influence of substituents in macrocyclic ligands derived from 18-crown-6 reflected in decreased amount of transported Cd(II) ions, compared to 18-crown-6 (61,26%), in all used solvents, following the order: DCH18C6<DB18C6<B18C6<18C6. Addition of nonionic surfactants as possible carriers for Cd(II) ions within the liquid membranes also resulted with decreased transport rates, due to additional interactions of Cd(II) ions and carriers within the membrane.
Keywords: Liquid membrane, Cd(II) ions, Surfactants, Macrocyclic ligands
Cite this paper: Mersiha Suljkanović, Edita Bjelić, Jasmin Suljagić, Azra Kovačević, Application of Different Ligands to Optimize Cd(II) Removal Through Liquid Membranes, Advances in Analytical Chemistry, Vol. 10 No. 1, 2020, pp. 7-10. doi: 10.5923/j.aac.20201001.02.
Article Outline
1. Introduction
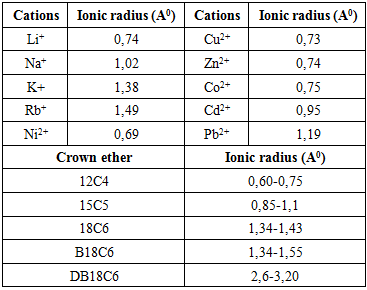
- Since the interactions between metal cations and ligands ("host-guest" interactions) depend on many factors, resulting stability of the formed complex can be increased by varying experimental conditions during transport experiments, e.g. during complex formation in an organic solvent as a membrane. One of the most important factors influencing complex formation is the compatibility between "the host" molecule (ligand) and "the guest" (metal ion). Polyether ligands are among the most suitable host molecules for many metal ions, due to the presence of oxygen atoms as electron donors in their structure, which enables formation of a coordinate covalent bond. Crown ethers have the specific macrocyclic structure of their molecules, consists of the polyether chain-forming "the crown" with hydrophilic cavity and hydrophobic surface.Macrocyclic structure of these ligands provides high stability of formed complexes with metal ions due to the "macrocyclic effect" [1].The affinity of the ligand to the metal ion depends on the structural characteristics of the ligand, and primarily on: the number and type of donor atoms, polarity, the hydrophilic-lipophilic balance of the ligand, chirality and other stereochemical indicators. The cations that best fit the cavity are located in the center and optimize interactions with heteroatoms (oxygen atoms).
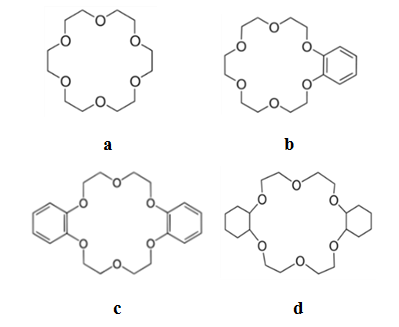 | Figure 1. Structures of polyether ligands: macrocyclic "crown ethers" a) 18-crown-6; b) benzo-18-crown-6; c) dibenzo-18-crown-6; d) dicyclohexano-18-crown-6 |
|
2. Material and Methods
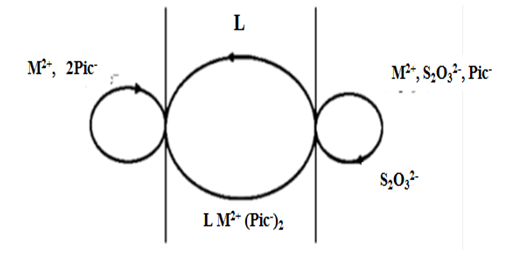
- MaterialFor every transport experiment, two aqueous solutions and one non-aqueous organic solution (membrane), were prepared, as follows. Source Phase solutions were prepared using the AAS standard metal ion solutions (Cd) from Merck, and picric acid (99%, Kemika) and adjusted to pH = 5, using the acetic buffer solution. Membrane Phase solutions were prepared by dissolving different macrocyclic ligands: 18-crown-6, 18C6; benzo-18-crown-6, B18C6; dibenzo-18-crown-6, DB18C6; dicyclohexano-18-crown-6, DCH18C6 (99%, ACROS ORGANICS) and non-ionic surfactants: Triton X-100, Brij 35, Brij 58, Brij 78 (p.a. Sigma-Aldrich) in different organic solvents (p.a. Kemika): dichloromethane (DCM), chloroform (CH), 1,2-dichloroethane (12-DCE). Receiving Phase solutions were prepared also in acetic buffer medium (pH = 5), by dissolving sodium thiosulphate (p.a. Sigma-Aldrich) in it.InstrumentspH measurements of aqueous solutions were performed using the pH meter (GLP31 Crison Instruments).Quantification of metal ions removed during the transport experiments were obtained by Flame Atomic Absorption Spectrometry technique, using the instrument Perkin Elmer AAnalyst 200. Transport ProcedureTransport experiments involved the application of a cylindrical glass vessel, "transport cell" (Figure 2.), with an inner diameter of 5 cm, containing a glass tube (2 cm in diameter) in a central position. The central tube represents a physical barrier between the two aqueous phases. The source phase (SP) contained 10 mL of a mixture of tested metal ion (1∙10-3mol/L) and the counter ion, picrate (1∙10-3 mol/L). The receiving phase (RP), which is outside the central tube, contained a stripping agent (thiosulphate). The membrane phase (MP) contained 50 mL of a suitable ligand (1∙10-3mol/L) dissolved in an organic solvent; the membrane layer lies beneath the aqueous phases and connects them. The membrane phase is mixed with a magnetic stirrer so that under these conditions the contact surfaces between the aqueous phases are straight and precisely defined [5]. Both aqueous phases were analyzed by atomic absorption spectrometry after 3hours, and the concentration of metal ions transported through the membrane was measured.
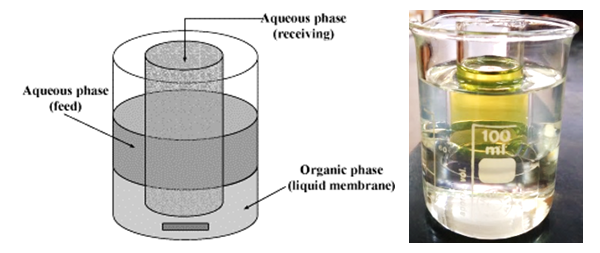 | Figure 2. "Transport cell" [6] used for the experiment: SP-source phase; RP-receiving phase; MP-membrane phase; A-magnetic stirrer |
3. Results and Discussion
- In earlier investigations, authors [7], [8] assumed that the most influencing process during transport experiments was metal ions release from complex in membrane phase to receiving aqueous phase through contact surface between two phases. Based on this assumption, the mechanism for metal ion transport was proposed [7].
3.1. Macrocyclic Carrier Study
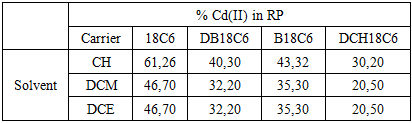
- BLM systems with membrane phase containing different macrocyclic ligands, (18C6; DB18C6; B18C6 and DCH18C6) was examined using different liquid membranes: CH, DCM and 1,2-DCE. CH membrane with 18C6 as a carrier showed better transport efficiency, for all ligand systems investigated (Table 2).
|
3.2. Study of Surfactants as Additional Carriers
- The possibility of using nonionic surfactant TX-100 as carrier for Cd(II) ions in liquid membrane systems has been investigated (Figure 4.) TX-100 addition resulted with the content of Cd (II) ions in the RP of 52%, which is lower compared to the system with only 18C6 (>60%). Comparation between systems using other macrocyclic ligands with addition of TX-100, showed also the reduction of transport in the RP. Stronger interactions within the membrane (cation-18C6-surfactant) may result in difficult cation release at the MP / RP boundary, either due to the formation of micellar structures in the membrane (incorporating the formed complexes), or due to the additional complexation of the cation by the surfactant as a ligand.
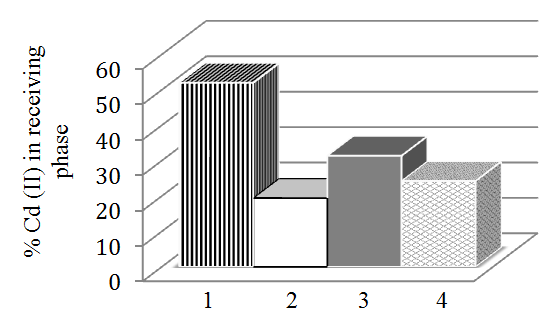 | Figure 4. Comparation of Cd(II) transport efficiency through CH membranes for different ligands: 1 –18C6+TX100; 2- DB18C6+TX100; 3–B18C6+TX-100; 4-DCH18C6+TX100 |
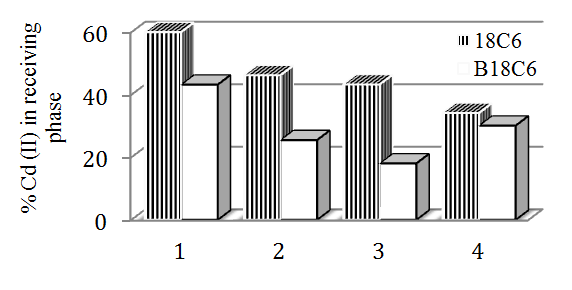 | Figure 5. Comparation of Cd(II) transport efficiency through CH membranes for different ligands: 1 –18C6; B18C6 2 –18C6+Brij78; B18C6+Brij78; 3- 18C6+Brij 58; B18C6+Brij58 4-18C6+Brij35; B18C6+Brij35 |
4. Conclusions
- 18-crown-6 ether is the most suitable for complexation of Cd(II) ions compared to dibenzo-18-crown-6, benzo-18-crown-6 and dicyclohexano-18-crown-6 due to its greater flexibility. Presence of nonionic surfactants TX-100, Brij 35, Brij 78 and Brij 58 with crown ether in membrane decreases transport efficiency due to incorporation of formed complexes into the micellar structures (reverse micelles) which stabilize them, preventing decomplexation and metal ions releasing from membrane to receiving phase.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML