-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2020; 10(1): 1-6
doi:10.5923/j.aac.20201001.01

Facile Root for Isolation of Rhamnocitrin Sulphate from Tetracera Alnifolia Willd
Victor N’Goka 1, 2, 3, Nicaise Narcisse Obaya 2, 3, Jean Bruno Mokoko 3, 4, Cyril Antheaume 5
1Department of Chemistry, Pharmacochemical and Pharmacotechnical Laboratory of Medicinal Plants, Chired, Congo
2Faculty of Science and Technology
3Marien Ngouabi University, Brazzaville, Congo
4Masters Department, Faculty of Science and Health, Pharmacology and Biochemistry Laboratory
5Laboratory of NMR, LC1 IFR85, ULP, Faculty of Pharmacy, Strasbourg, France
Correspondence to: Jean Bruno Mokoko , Marien Ngouabi University, Brazzaville, Congo.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Rhamnocitrin 3-sulphate has been isolated with one percent yield, from Tetracera alnifolia, a non-cultivated medicinal plant from Congo. Rhamnocitrin 3-sulphate has been isolated after recrystallization of crude dried extract obtained from aqueous decoction of Tetracera leaves and stem. In this paper, Rhamnocitrin 3-sulphate has been easily isolated for the first time from Tetracera alnifolia and the corresponding tri-acetylated compound has been synthetized. Identification, characterisation and structure elucidation have been carried out using TLC, HPLC, UV-VIS, NMR and MS analyses in comparison with those described in the literature.
Keywords: Rhamnocitrin 3-sulphate, Rhamnocitrin, Tetracera alnifolia, Dilleniaceae, Flavonol
Cite this paper: Victor N’Goka , Nicaise Narcisse Obaya , Jean Bruno Mokoko , Cyril Antheaume , Facile Root for Isolation of Rhamnocitrin Sulphate from Tetracera Alnifolia Willd, Advances in Analytical Chemistry, Vol. 10 No. 1, 2020, pp. 1-6. doi: 10.5923/j.aac.20201001.01.
Article Outline
1. Introduction
- Nowadays World Health Organization (WHO) exhorts African States to produce traditional ameliorated medicine. However, the development of these products has been subordinated to the standardisation of the procedure for their elaboration. This paper describes the isolation of Rhamnocitrin 3-sulphate with high amount in one step, instead of the traditional multistep procedure of isolation of molecule form vegetable matter as shown by Yamauchi et al. and Chaabi et al. for examples [1,2]. Tetracera alnifolia Willd is a perennial, evergreen big liana of the family of Dilleniaceae. It is growing wildly in the forest and most warm regions of the world especially in Senegal, Central African Republic, Gabon, Cameroon, Congo, Congo RD, Angola, Fernando Po and Zambia. T. alnifolia leaves and stem have been traditionally used for treating venereal diseases, abdominal pains and to regulate menstruation [3,4], which indicates probably the presence of antibacterial compounds in this plant. Preliminary antibacterial study of T. alnifolia have been reported and showed substantial activity against Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus faecalis, Echerichia coli, Shigella dysenteriae, Yersinia enterocolitica, Enterobacter cloacea, Salmonella sp. abony, Serretia marcescens and Pseudomonas aeruginosa [5]. Nowadays T. alnifolia is used in association with other medicinal plants to make traditional ameliorated medicine sold in Congolese pharmacies; but the chemical composition of this antimicrobial and anti-inflammatory preparation is unknown. In our knowledge no chemical composition of T. alnifolia extract has been reported. Rhamnocitrin, a 7- methyl-Kaempferol (Figure 1), has been identified in many plants [6-10] and this interesting compound has been isolated as the corresponding triacetoxy derivative in little amount, 35 mg by Rossi et al. [8]. Rhamnocitrin has been reported to show several biological activities. It has antibacterial, anti-inflammatory effect [11], including strong inhibition of platelet aggregation induced by arachidonic acid [12]. Its anti-inflammatory and antispasmodic activities have been reported [13] as well as its strong antioxidant activity [11]. Qualitative studies of flavonoid sulphates have been well documented and particularly Rhamnocitrin 3-sulphate has been identified from Tetracera poggei Gilg and T. alnifolia by Gurni et al. [14,15]. Silva and colaborators [10] described Rhamnocitrin by long and expensive usually method of extraction and isolation from Solanum jabrense and S. Paludosum. In the present work we report an easy and cheap method for quantitative isolation and purification of Rhamnocitrin 3-sulphate from T. alnifolia using crystallization only. The molecular formula was elucidated on the basis of spectroscopic studies in comparison with those described in the literature.
2. Materials and Methods
- 1. Plant MaterialLeaves and stem of T. alnifolia Willd have been collected near MASSENGO area, in the north of Brazzaville, Congo. The plant sample was kindly authenticated by comparison with a voucher specimen at the Herbarium of the “Centre de Recherche sur les Ressources Végétales (CERVE)” in Brazzaville, Congo. 2. Preparation of the crude extract from T. alnifolia Willd Dried powdered, 25 g, of leaves and stem of T. alnifolia were boiling in 300 ml of water for 30 min with shaking. The remaining solution has been filtered using Whatman paper n° 40; 8 µm. The filtrate solution has been concentrated in vacuum at 40°C still dryness to give a Rhamnocitrin 3-sulphate rich extract. And then, 250 mg of pure Rhamnocitrin 3-sulphate, has been obtained after crystallization of the above dry crude extract in water. Experience was carried out in triplicate. The yield of Rhamnocitrin 3-sulphate was of 1% w/w from dried vegetable material.
3. Experimental Equipment
- NMR spectra were recorded with a Bruker Avance DPX-300 spectrometer (at 300 MHz for 1H and 75 MHz for 13C NOESY spectra were obtained at 400 MHz. Mass spectra were taken on a ESI Bruker Daltonics HCT ion trap mass spectrometer. The UV spectra were obtained on a HPLC system: pump: VARIAN 9010, injector: VARIAN 9100, detector: VARIAN Prostar 330 provided with a pin of diodes. TLC Plates, Silica gel Merck 60 F254, (20x20 cm) were used. The flavonoides bands were visualized by spraying with Dragendorff’s reagent, followed by 10% HCl, and or using ultraviolet light at 254 and 365 nm.
4. Results and Discussion
- Pure compound was isolated as a yellow amorphous powder, and the purity was confirmed by TLC, Rf = 0.46 (Ethyl acetate-methanol: 8-2); HPLC and SM (Figure 1 and 2). The structure (Figure 3) was elucidated by comparison of the spectroscopic data with the corresponding literature values for the un-sulphated compounds [9,10,17-21]. The ESI-MS and EI-MS have been analysed by comparison of the previously described for Kaempferol [20]. The product ion mass spectrum for Rhamnocitrin 3-sulphate m/z 380 is shown in figure 2. A peak at m/z 379 [M-H] was consistent with a molecular formula C16H12SO9-H; m/z 315 [M-H-SO2] corresponding to the loss HSO2; m/z 299 [M-HSO3] corresponding to the loss of HSO3; m/z 284 [M-HSO3-CH3] corresponding to the demethylated of later product; m/z 271 [M-HSO3-C=O] corresponding to the loss of HSO3 and C=O; m/z 255 [M-HSO3-CH3-HC=O] corresponding to the loss of HSO3, HC=O and demethylation; m/z 243 [M-HSO3-CH3-CH=C=O] corresponding to the loss of HSO3, CH=C=O and demethylation; m/z 227 (M-HSO3-OCH3-CH=C=O] corresponding to the loss of HSO3, CH=C=O and demethoxylation.The 13C NMR and DEPT spectrum (Figure 4) showed signals due to 16 carbons comprising one methoxyl, six aromatic methine, nine quaternary carbons from where; two fully substituted aromatic carbons, two oxymethine, one bearing a carbonyl and four bearing an oxygen atom from where two similar bearing hydroxyl, one bearing sulphate and the last for the methoxyl. These fragments account for a molecular formula C16H12SO9, indicating that the two remaining hydrogen atoms are present in hydroxyl groups. The 1H NMR spectrum (Figure 5) showed signal at δ 3.86 ppm typical of methyl ether (OMe) by three proton singlet. Also in 13C NMR spectrum the presence of methyl ether group was brought in evidence by the signal observed at δ 56. In the 1H-1H NOESY (Figure 6A) contour plot the methine H-2’and H-6’ showed correlations to both H-3’and H-5’ which confirmed the presence of the 1’, 4’- substituted phenyl fragment. The presence of a methoxyl group at C-7 as shown in figure 6B was confirmed by the nOe correlations between the proton in C-7 and the protons in C-6 and C-8 (Figure 6 and figure 7). The presence of a sulphate group at C-3 in the latter compound was confirmed by the signal found at δ 156.6 instead of δ 136 for the un-sulphated Rhamnocitrin compound. The 1H NMR spectrum of acetylated compound showed three acetoxyl group at δ 2.41, 2.32, 2.30 besides signal characteristic of methoxyl group at δ 3.89 and signals of protons bearing by carbons C-6 and C-8 became doublet with allylic J=2.4 Hz showing meta position of theirs protons. Comparisons between acetylated and non acetylated compound allowed the assignment of the positions of the acetoxyl and methoxyl groups. In the acylated compound, the signals of both H-2’ and H-6’ shift upfield for 0.71 ppm this is in accordance with the OAc in C-3 position (Rossi et al., 1997), the signals for H-3’ and H-5’ move 0.37 ppm downfield this is in accordance with the OAc at C-4’. We observed a little diamagnetic effect for the signal of H-6 and H-8. Signals of H-6 and H-8 have been moved downfield only for 0.10 ppm in the acetylated product. But our 1H-NMR result was the same as that previously described by Silva et al. 2009 and Scio et al. 2003 [9,10,19], for Rhamnocitrin and by Rossi et al., for three acetylated compound [8]. These inferences and a detailed analysis of the 1H-NMR, 1H-1H NOESY, HMQC, and HMBC spectrum and comparison with literature data suggested a Rhamnocitrin 3-sulphate.Rhamnocitrin 3-sulphate was isolated as a yellow amorphous powder, (250 mg); UV λ max (MeOH) nm: 340, 265; 1H NMR (DMSO; 300 MHz); 8.13 (2H, d, J = 8.9, H-2’ and H-6’), 6.85 (2H, d, J=8.9, H-3’ and H-5’), 6.71 (1H, s, H-8), 6.34 (1H, s, H-6), 3.86 (3H, s, OCH3 -7). 13C NMR (DMSO; 75 MHz) 177.8 (C-4), 164.9 (C-7), 161.0 (C-4’), 160.0 (C-5), 156.6 (C-3), 156.0 (C-9), 132.5 (C-2), 130.8 (C-2’ and C-6’), 121.1 (C-1’), 115.0 (C-3’ and C-5’), 105.1 (C-10), 97.5 (C-8), 92.1 (C-6), 56,0 (OCH3). MS (70 eV) m/z C16H11SO9; 379 (M-H), 315 (M-H-SO2); 299 (M-H-SO3), 284 (M-H-SO3-CH3); 271 (M-H-SO3-C=O); 255 (M-H-SO3-CH3-HC=O); 243 (M-H-SO3-CH3-CH=C=O); 227 (M-H-SO3-OCH3-CH=C=O).3,5,4’-Triacetoxy-7-methoxyflavone or 3,5,4’-Triacetoxy-Rahmnocitrin. UV λ max (MeOH) nm: 304, 252; 1H NMR (300 MHz, CDCl3): 7.82 (d, J = 8.7, H-2’, H-6’), 7.22 (d, J = 8.7, H-3’, H-5’), 6.82 (d, J= 2.4, H-8), 6.43 (d, J = 2.4, H-6), 3.89 (s, OMe), 2.41 (s, OAc), 2.32 (s, OAc), 2.30 (s. OAc).
5. Conclusions
- The study has demonstrated to be very important particularly for the production scale of Rhamnocitrin 3-sulphate. Recristallization is very simple, easy, cheap and perfect for isolation of pure Rhamnocitrin 3-sulphate from Tetracera alnifolia which could be considered as raw material for several usages.
ACKNOWLEDGEMENTS
- This study is carried out under the program of Chimie Recherche et Développement Pharmaceutique (CHIRED-CONGO). The authors are deeply indebted to Madame Kouka of the CERVE for plant identification.
Annexes
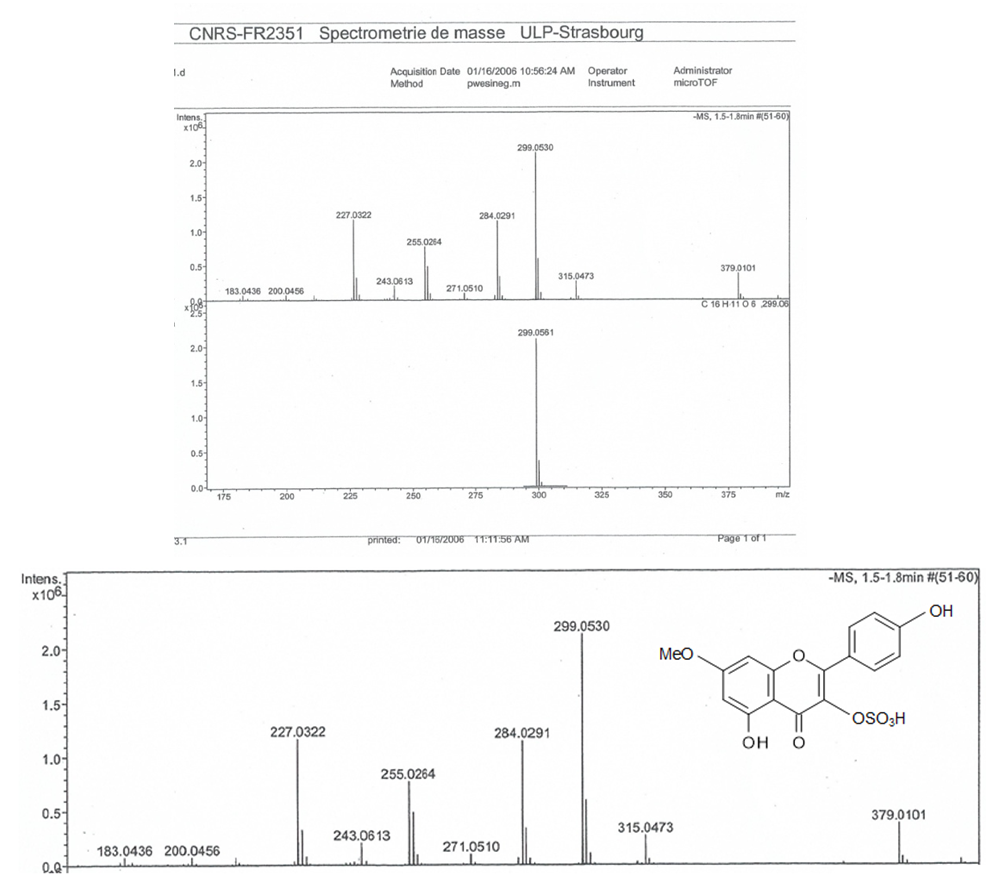 | Figure 2. MS Spectra of Rhamnocitrin 3-sulphate |
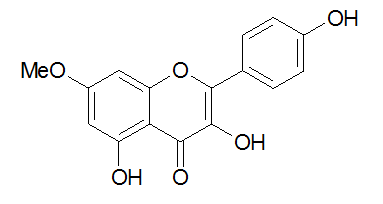 | Figure 3. Rhamnocitrin = 7- methyl-Kaempferol |
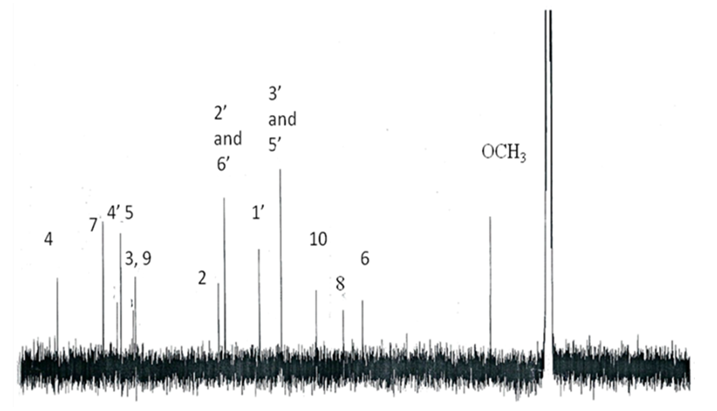 | Figure 4. NMR 13C Spectra of Rhamnocitrin 3-sulphate |
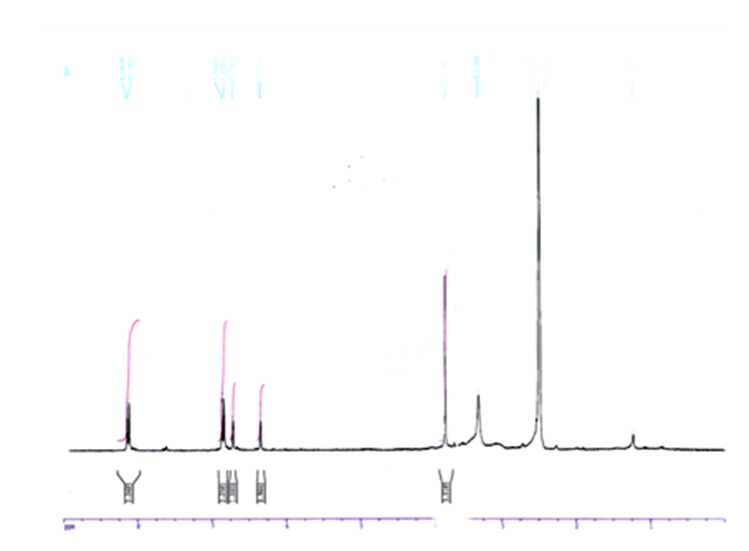 | Figure 5. NMR 1H Spectra of Rhamnocitrin 3-sulphate |
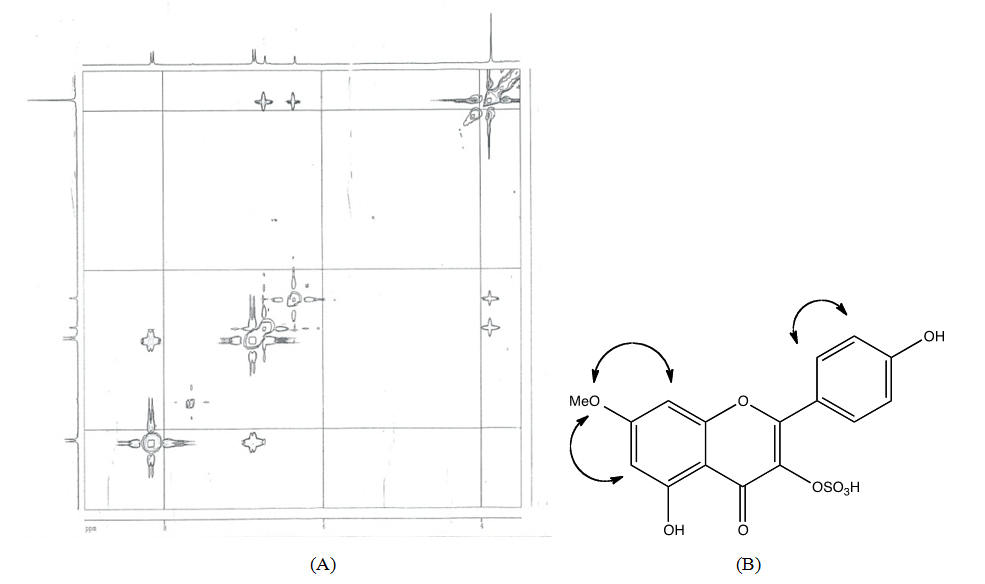 | Figure 6. (A) 1H-1H NOESY-NMR Spectra of Rhamnocitrin 3-sulphate, (B) nOe key NMR correlations of Rhamnocitrin 3-sulphate |
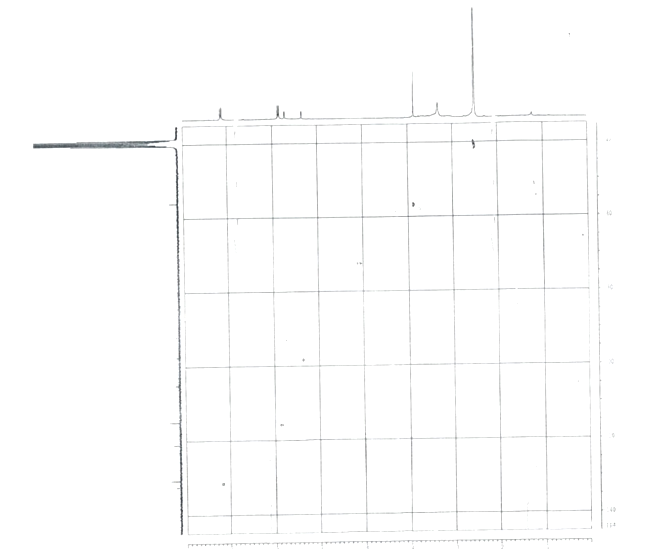 | Figure 7. 1H-13C HSQC NMR Spectra of Rhamnocitrin 3-sulphate |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
 Phytochemistry. 1972, 11(7): 2313-2316.
Phytochemistry. 1972, 11(7): 2313-2316.