-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2019; 9(2): 34-37
doi:10.5923/j.aac.20190902.02

Phytochemical Screening and Antibacterial and Antifungal Activity of allium sativum Juice on Multi-Resistant Strains
Mokoko Jean Bruno 1, 2, Miguel Landry 1, 2, Mbemba Bahamboula Destiny 1, 2, Mouankie Jean Bertin 1, 2, Abena Ange Antoine 1, 2
1Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Republic of Congo
2Laboratory of Pharmacology and Biochemistry
Correspondence to: Mokoko Jean Bruno , Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Republic of Congo.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Among these plants are the species of the genus Allium which are unequally distributed in the world. There are more than 120 documented uses, but its chemical identity and antibacterial and antifungal properties (Agarwal K.C. et al. 1996,) are very poorly documented in the Congo. Material and Methods: We used fresh bulbs of Allium sativum grown in Congo, after grinding and filtration of garlic 120 ml of juice was obtained after. Phytochemical studies were carried out with determination of the content of flavonoids and on a young culture the antibacterial and antifungal activity in vitro of the aqueous extract of garlic was evaluated in the Bacteriology department, of the National Public Health Laboratory. Result: Allium Sativum harvested in Congo has a strongly positive flavonoid level. Gram-positive bacterial strains were more sensitive to the extract than gram-negative bacterial strains. The inhibitory effect of pure allium sativum extract was effective compared to conventional antibiotics. Conclusion. This study allowed us to justify the validity of the antimicrobial virtues granted to this plant.
Keywords: allium sativum, Phytochemistry, Antibacterial, Antifungal
Cite this paper: Mokoko Jean Bruno , Miguel Landry , Mbemba Bahamboula Destiny , Mouankie Jean Bertin , Abena Ange Antoine , Phytochemical Screening and Antibacterial and Antifungal Activity of allium sativum Juice on Multi-Resistant Strains, Advances in Analytical Chemistry, Vol. 9 No. 2, 2019, pp. 34-37. doi: 10.5923/j.aac.20190902.02.
Article Outline
1. Introduction
- Antibiotic resistance is a natural and predictable mechanism that refers to a situation where an antibiotic that should normally have stopped the development of a certain type of bacteria is no longer able to do so (Acar J.F et al., 2003). According to the WHO, with no response from the international community, more than 10 million people could die worldwide each year from resistant bacterial infections in 2050 (WHO, 2015). In Africa, many cases of multi-resistance have been reported particularly to beta-lactamases with rates ranging from 30% to 50% in enterobacteria or for resistance to meticillin in Staphylococcus aureus which is greater than 30% (Ouedraogo et al., 2017). In Congo, one study reported the prevalence of H. pylori infection of 75.52%, resistant to clarithromycin (4.2%) and tetracycline (1.2%) and levofloxacin (57%) (Ontsira et al., 2016). One solution is to explore medicinal plants, whose antibacterial and antifungal potency is believed to be due in whole and/or in part to the substances they contain (Dorant et al., 1996). Among these plants are the species of the genus Allium which are unequally distributed in the world. With each according to the location recovered the difference of taste, shape and color, but the biochemical identity remains close. There are more than 120 differents documented uses, however its antibacterial and antifungal properties (Adetumbi et al., 1993) are very poorly documented in the Congo. For example, the purpose of this study is to identify the different substances in the aqueous extract of garlic from Brazzaville, as most of its therapeutic properties are attributed to allicin (Sendl, 1995; Jiben et al., 2006) and to assess in vitro the antibacterial and antifungal potency of Allium sativum juice vis-à-vis «multi-resistant» clinical strains.
2. Materials and Methods
2.1. Plant Material
- We used the fresh bulbs of Allium sativum grown in Congo and present in the markets of Bacongo, Brazzaville (Congo) from 1 to 5 September 2018 and have been kept at room temperature at the Laboratory of Pharmacology and Biochemistry of the Faculty of Health Sciences.
2.2. Preparation of Garlic Juice Extract
- We obtained 120 ml of juice after grinding and filtration of garlic and their cloves; the filtrate was then centrifuged at 3000 rpm for 20 minutes and the supernatant was recovered and kept at +4°C until use. To assess activity, dilutions of the juice in physiological water were prepared (100%, 80%, 50%, 20%, 10%).
2.3. Preparation of Solid Extractions
- We impregnated the blotting paper discs (wattman) with garlic juice, of each previously prepared concentration for (07) seven hours.
2.4. Phytochemical Screening
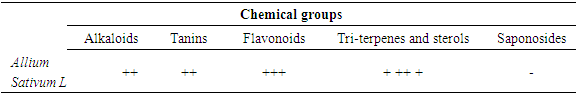
- This is a technique that allows the presence of groups of chemical families in active extracts of a plant substance to be demonstrated in order to evaluate the anti-bacterial activity. The results were presented as follows: (+++) Strongly positive, (++) Moderately positive, (+) Weakly positive and (- ) Negative. 2.4.1. Flavonoids: Two (2) ml of the acquired extract have been evaporated and 5 ml of residue has been recovered in summer mixed with a few drops of magnesium, there is a heat release then a yellow coloration. this colouration confirmed the presence of flavonoids [Azzi R, 2012]. The Aluminum Trichloride method is used to quantify flavonoids in the extract. Absorbance is read at 415nm by a UV-visible spectrophotometer.2.4.2. Tannins: Highlighted by adding to 1 ml of water extract, 1 ml of water and 1 to 2 drops of 1% diluted Fecl3 solution The appearance of different colours corresponds to a specific type of tannin. 2.4.3. Saponins: a series of 5 numbered tubes, 10ml of the solution to be analysed prepared by decoction in an aqueous medium is introduced followed by an adjustment of the with distilled water. Shake each tube lengthwise for 15 seconds at 2 agitations per second. Let stand 15 min and measure the height of the foam produced in each tube. 2.4.4. Tri-terpenes and Sterols: they are sought by the Liebermann reaction. 2.4.5. Alkaloids: In order to check their presence, a precipitation reaction with Dragendorff reagent is required. [Azzi R, 2012].
2.5. Selection of Microbial Strains
- To assess the antimicrobial activity of allium sativum, clinical strains with lethal resistance of at least four (04) conventional antibiotics and/or antifungals were selected. Micro organisms from urine (Candida.albicans, Candida.spp, Staphylococcus.aureus, Klebsiella.oxytoca, Enterobacter.spp), stool (Eschéricha.coli) and vaginal collection (Staphylococcus. non coagulase Klebsiella.pneumonia).
2.5.1. Antimicrobial Activity
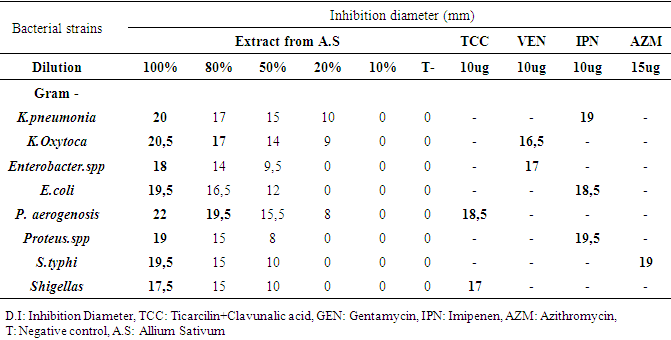
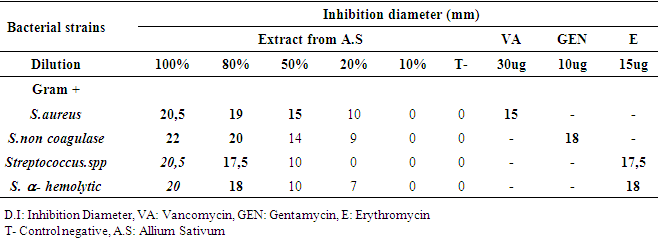
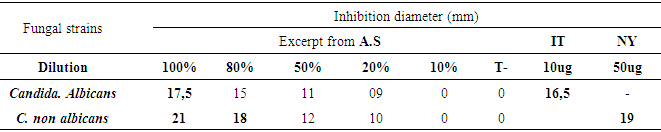
- The method of diffusion on disk was used from 19 to 22 September 2018 to evaluate the antibacterial and antifungal activity in vitro of the aqueous extract of garlic in the department of Bacteriology, of the National Laboratory of Public Health. On a young crop, we sampled a colony, suspended 10ml of physiological water, and then inoculated on the surface of a Muller Hinton agar can (MH) by swabbing. Then, sterile blotting paper discs (Wattman N°1, 6 mm in diameter) impregnated with each garlic juice dilution and an additional disc reserved for the negative control (distilled water) were placed with a sterile clamp on the surface of the M.H petri dishes previously seeded with the bacterial and fungal strains to be tested. Petri dishes were left at 37°C for 30 minutes to allow the juice to be distributed. They were then incubated at 37°C for 18-24 h. Inhibition diameters were expressed in mm. The tests were duplicated and the results were expressed as averages.The T Student test was used to calculate the mean inhibition diameters of bacterial and fungal strains.
3. Results and Discussion
3.1. Phytochemical Study
- The presence in strong or small quantities of each chemical fraction was judged by the degree of staining:
|
 | Figure 1. Result after mixing |
3.2. Anti-microbial Activity
|
|
3.3. Antifungal Activity of Garlic Juice
|
4. Conclusions
- The chemical identity of allium sativum grown in the Congo is very close to that described in the literature. The sensitivity of the different strains tested to allium sativum juice and the antibiotic and/or fungal control is of great importance in the treatment of the associated pathologies. This study allowed us to justify the validity of the antimicrobial virtues granted to this plant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML