-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2019; 9(1): 12-22
doi:10.5923/j.aac.20190901.03

Pitting Corrosion of Aluminum in Aqueous Solution with Low pH Containing Nitrate Ion and Its Inhibition Using Different Surfactants
O. A. Abdullatef1, W. A. El-Mahmoudy2
1Faculty of Pharmacy, Pharos University, Alexandria, Egypt
2Faculty of Science, Alexandria University, Alexandria, Egypt
Correspondence to: O. A. Abdullatef, Faculty of Pharmacy, Pharos University, Alexandria, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Localized or pitting corrosion of aluminum in acidic medium is a problem for some industries. Severe attack can lead to pinhole leaks and instrument breakdown. The results showed that pitting corrosion occurred in waters with low nitrate ion concentration. The effect of adding different types of surfactants on the inhibition of the pitting corrosion of aluminum in 0.1 M HNO3 was studied using potentiodynamic polarization and electrochemical impedance (EIS) spectroscopy techniques. The electrochemical results proofed that both the anionic surfactant sodium lauryl sulfate and the cationic surfactant cetrimide can decrease the pitting corrosion of aluminum in aqueous solution containing nitrate ion to a great extent. The mechanism of adsorption of surfactants on metal surface was studied using the kinetic thermodynamic model. The free energy change of adsorption indicated that the adsorption of surfactants on metal surface is spontaneous in nature. The adsorption of both surfactants is comprehensive process (physical and chemical).
Keywords: Pitting corrosion, Surfactant, Electrochemical, Physical Adsorption
Cite this paper: O. A. Abdullatef, W. A. El-Mahmoudy, Pitting Corrosion of Aluminum in Aqueous Solution with Low pH Containing Nitrate Ion and Its Inhibition Using Different Surfactants, Advances in Analytical Chemistry, Vol. 9 No. 1, 2019, pp. 12-22. doi: 10.5923/j.aac.20190901.03.
Article Outline
1. Introduction
- It is not surprising that aluminum and aluminum alloys are used through the world due to their high technological value and their use in industrial applications especially in household and aerospace industries [1-3]. Aluminum and its alloys are economically important due to their light weight, high electrical capacity and good corrosion resistance [4, 5]. Despite the fact that aluminum is a reactive metal, it is resistant to corrosion in solutions of moderate pH ranged from 4 to 9, only in absence of aggressive ions as chlorides and sulfates [6, 7]. The resistance of aluminum to corrosion is a result of the formation of a thin, well adhered and protective oxide layer [8, 9]. Far away from the pH range 4 to 9, the oxide film is soluble and promote uniform attack [6, 10].The behavior of aluminum and its alloys in aggressive environments have been studied [10-14]. Acid pickling of aluminum is widely used in the chemical industries to remove the scales from the metallic surface [15]. Many chemical inhibitors are used to prevent the dissolution of aluminum surfaces during its chemical etching [16, 17]. The organic compounds containing hetero atoms such as sulphur, nitrogen or oxygen in their structure are the most effective corrosion inhibitors [18-21]. Finding new inhibitors for corrosion of aluminum is still understudy. The pitting corrosion of aluminum usually occurs in aqueous solutions in presence of aggressive ions such as Cl- [22-27], ClO3- [28], ClO4- [28-31] and SCN- [32] anions. It is said that pitting corrosion of aluminum can be described by the local break down of the passive oxide film. The pitting corrosion of aluminum in aluminum-electrolyte system is characterized by a critical break down potential Eb resulting in break down of the oxide passive film [22].Surfactants are commonly used chemicals in many industrial applications [33]. Nowadays, Surfactants play a new role in corrosion inhibition technology [34-38]. The advantages of surfactants over other chemical inhibitors are their cheapness, easy production and their low toxicity [39]. The mechanism of action of surfactants on the metal surface is its adsorption in such a way that the polar part (hydrophilic) attaches the metal surface. On the other hand, the non-polar part (hydrophobic) extends in the solution [40]. The adsorption of surfactant on the metal surface depends on the structure of the surfactant [35, 41, 42]. Li et al, [43] studied the effect of tetradecylpyridinium bromide (TDPB) as corrosion inhibitor for aluminum in 1.0 M HCl solution by using weight loss, potentiodynamic polarization, and electrochemical impedance spectroscopy (EIS) techniques. The results showed that TDPB acts as cathodic-type inhibitor where its efficiency increases with increasing its concentration, and were found to be inversely proportional to the temperature. The adsorption of TDPB on aluminum surface is consistent with Langmuir adsorption isotherm. The inhibition effect of a 3-(10-sodium sulfonate decyloxy) aniline monomeric surfactant and the polymeric surfactant poly[3-(decyloxy sulfonic acid) aniline] (PC10) on the corrosion of aluminum in 0.5 M hydrochloric acid was studied by using weight loss and potentiodynamic polarization techniques [44]. The results showed that both surfactants acted as mixed-type inhibitors in which their inhibition efficiency increased with increasing surfactant concentration and decreased with increasing temperature. Mehdaoui et al, [45] synthesized some anionic surfactants [gasoil sulfonate (GOS), kerosene sulfonate (KES), heavy solvent sulfonate (HSS) and total gasoline sulfonate (TGS)] obtained from Algerian petroleum fractions and studied their effect on aluminum corrosion in hydrochloric solution (1 M HCl) using weight loss measurements, electrochemical polarization, and electrochemical impedance spectroscopy techniques. The results showed that the corrosion inhibition efficiency is in the order: TGS < HSS < KES < GOS which is in accordance with the number of carbon atoms in the hydrophobic chain of the inhibitor molecule. The electrochemical measurements showed that these surfactants act as cathodic inhibitors, and their adsorption on Al substrate is a physisorption-type, spontaneous and exothermic.According to our knowledge, there is no previous studies narrated that aluminum and its alloys undergo pitting corrosion in NO3- ions containing solutions. The aim of this study is to focus on the pitting corrosion of aluminum in nitric acid and to evaluate the different types of surfactants as corrosion inhibitors for aluminum in this media. The present study will be extended to assess their role in controlling the localized form of corrosion of aluminum.
2. Experimental
2.1. Electrochemical Measurements
- The electrochemical measurements were carried out by a frequency response analyzer potentiostat (ACM 604). The frequency range for impedance measurements (EIS) was 30 kHz to 0.01 Hz with applied potential signal amplitude of 10 mV around the rest potential. A three electrode mode cell was used, the cell contains an auxiliary graphite electrode and saturated calomel reference electrode. The working electrode was fabricated in cylindrical form. Aluminium was encapsulated in epoxy resin in such a way that only one surface was left uncovered. The working electrode has the chemical composition (% wt): Al 99.687; Mn 0.001; Zn 0.001; Ni 0.003; Fe 0.171; Si 0.135; Cu 0.001. The exposed area (0.5 cm2) of the sample was wet hand-polished using emery papers of variable grades starting with a coarse one and proceeding in steps to the finest (1000) grade. The sample was then washed thoroughly with double-distilled water and finally dried by absolute ethanol just before immersion in the solution. Each experiment was carried out with freshly polished electrode. Before polarization and EIS measurements, the working electrode was introduced into the test solution and left for 20 min at the open circuit potential. The polarization curve measurements were obtained at scan rate of 20 mV/min starting from cathodic potential (Ecorr -300 mV) going to anodic direction until 250 mV after the rest potential. All the measurements were done at 30.0 ± 0.1°C in solutions open to the atmosphere under unstirred conditions. To test the reliability and reproducibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.2. Solution Preparation
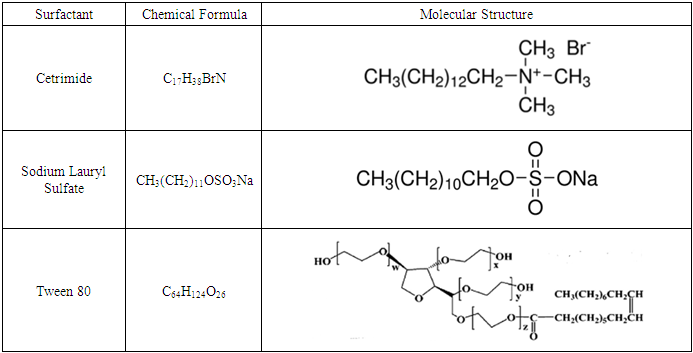
- The test solutions were prepared from analytical grade reagents and distilled water. 70% HNO3 was purchased from Aldrich Chemicals Company. Stock solution, of 1 M of HNO3 and 0.02 M of surfactant were used to prepare the test solution. Prior to each experiment, 1.0 M HNO3 is added to an appropriate volume of 0.02 M surfactant solution and double distilled water to obtain a solution of 0.1 M HNO3 and the required concentration of the surfactant. Tween 80, Cetrimide and Sodium Lauryl Sulphate were obtained from Alpha Chemica Company. Their molecular structures are given in Table 1.
|
3. Results and Discussion
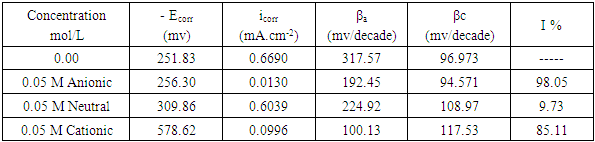
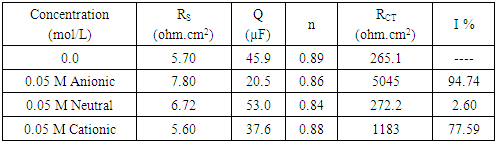
- In this study we were aiming to study the effect of different types of surfactants; neutral surfactant (Tween 80), anionic surfactant (Sodium Lauryl Sulfate, SLS) and cationic surfactant (Cetyl trimethyl ammonium bromide, Cetrimide) on the pitting corrosion of aluminum in 0.1 M HNO3, using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. In order to test this idea, the potentiodynamic polarization curves and the Nyquist impedance diagrams of aluminum in 0.1 M HCl were recorded in absence and presence of 0.05 M of each of the neutral surfactant (Tween 80), cationic surfactant (Cetrimide) and the anionic one (SLS). Figure 1 shows the polarization curves of aluminum in 0.1 M HNO3 solution in absence and presence of 0.05 M Tween 80, Cetrimide or SLS surfactants.
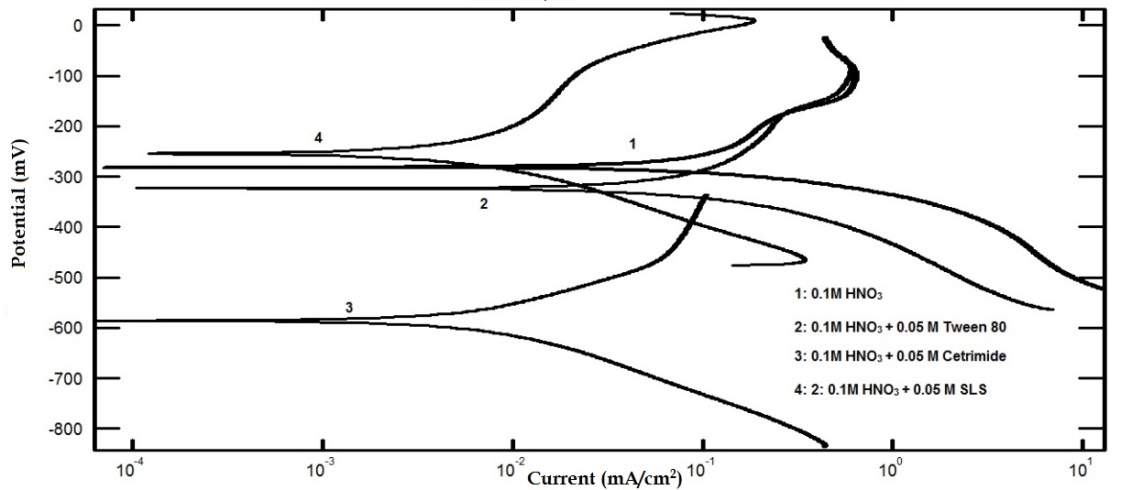 | Figure 1. Potentiodynamic polarization curves for aluminum in 0.1 M HNO3 solution in absence and presence of 0.05 M Tween 80, Cetrimide or SLS surfactants |
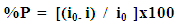 | (1) |
|
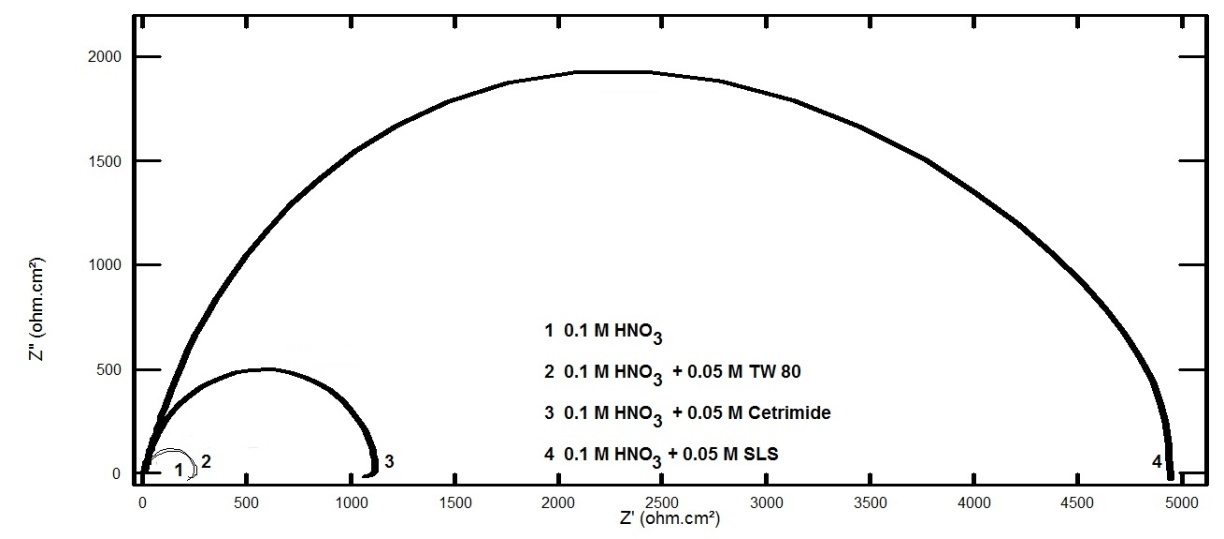 | Figure 2. Nyquist plots for aluminum in 0.1 M HNO3 solution in absence and presence of 0.05 M Tween 80, Cetrimide or SLS surfactants |
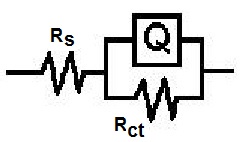 | Figure 3. Equivalent circuit |
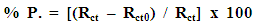 | (2) |
|
3.1. Inhibition of the Dissolution of Aluminum in 0.1 M HNO3 by Sodium Lauryl Sulfate (SLS)
3.1.1. Potentiodynamic Polarization Technique
- Figure 4 represents the potentiodynamic polarization curves for aluminum in 0.1 M HNO3 in absence and presence of different concentrations of SLS surfactant.
 | Figure 4. Potentiodynamic polarization curves for aluminum in 0.1 M HNO3 in absence and presence of different concentrations of SLS surfactant |
|
3.1.2. Electrochemical Impedance Spectroscopy
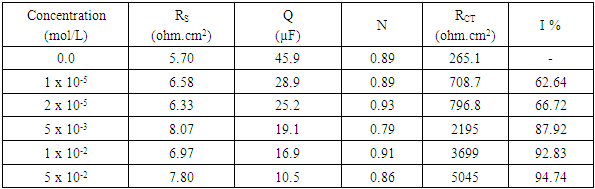
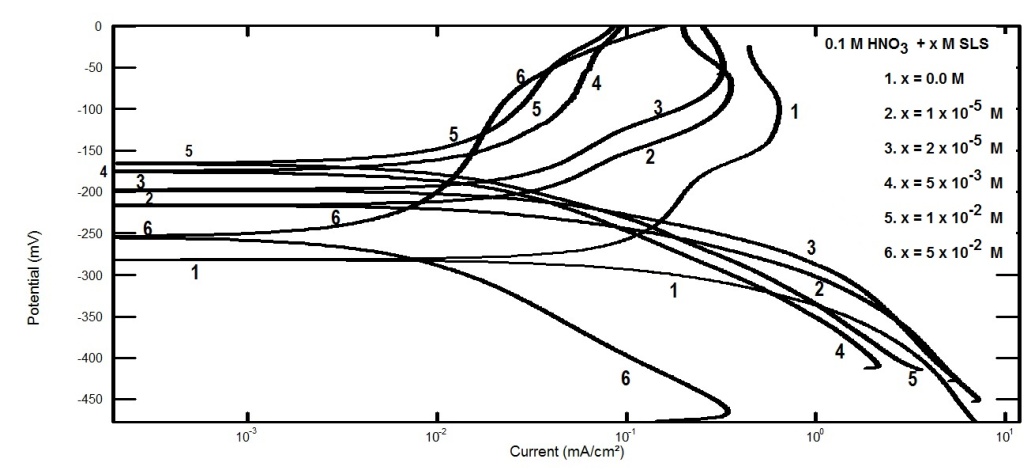
- Figure 5 represents Nyquist plots for aluminum in 0.1 M HNO3 in absence and presence of different concentrations of SLS.
 | Figure 5. Nyquist plots for aluminum in 0.1 M HNO3 in absence and presence of different concentrations of SLS |
|
3.2. Inhibition of Dissolution of Aluminum in 0.1 M HNO3 by Cetrimide
3.2.1. Potentiodynamic Polarization Technique
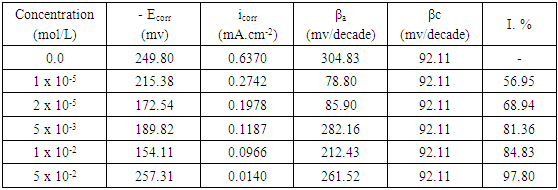
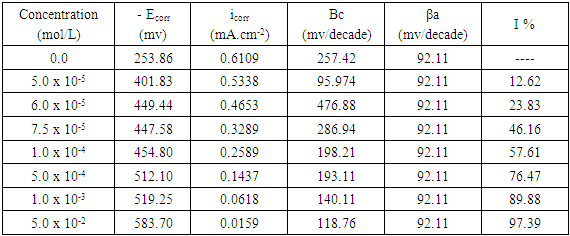
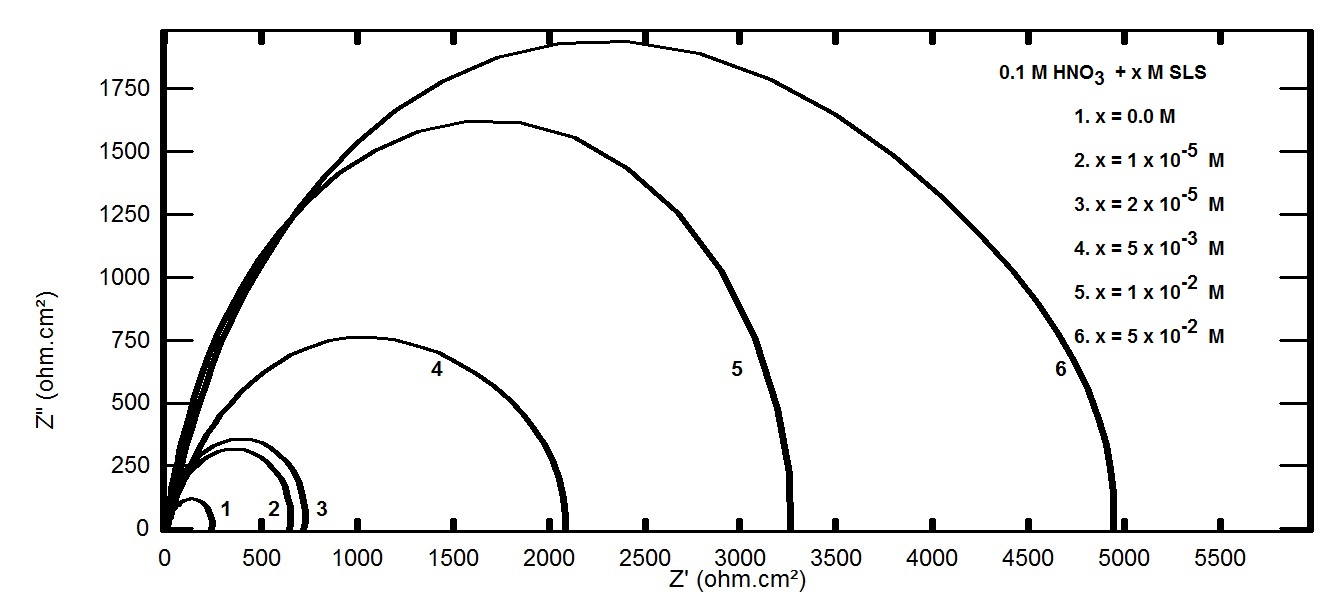
- Figure 6 represents the potentiodynamic polarization curves for aluminum in 0.1 M HNO3 in absence and presence of different concentrations of Cetrimide surfactant. Inspection of the figure shows that the presence of the cationic surfactant Cetrimide affect the equilibrium potential by shifting it to more negative potential. In the mean time, the presence of Cetrimide affects greatly the anodic polarization curves.
 | Figure 6. Potentiodynamic polarization curves for aluminum in 0.1 M HNO3 in absence and presence of different concentrations of Cetrimide |
|
3.2.2. Electrochemical Impedance Spectroscopy Technique
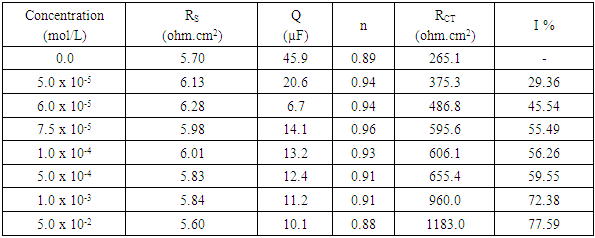
- Figure 7 represents Nyquist plots for aluminum in 0.1 M HNO3 in absence and presence of different concentrations of Cetrimide.
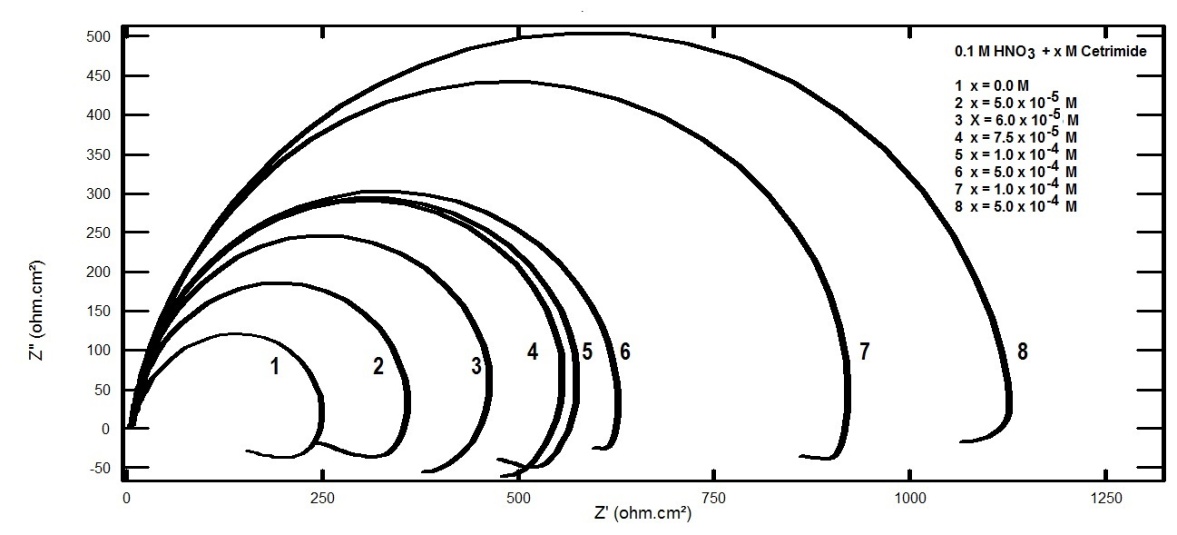 | Figure 7. Nyquist plots for aluminum in 0.1 M HNO3 in absence and presence of different concentrations of Cetrimide |
|
3.3. Mechanism of Adsorption of Surfactants
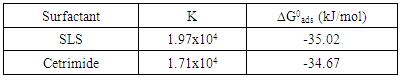
- It is known that surfactants have a tendency to associate at interfaces and in solution to form aggregates [49]. Adsorption of the surfactant molecules on to metal surface, which is the primary action of the surfactant functional group, was found to be responsible for the corrosion inhibition of the metal and is related to its capability to aggregate to form micelles [50-55]. The variation of the percentage inhibition, which was calculated from the polarization measurements, with different concentrations of both SLS and Cetrimide surfactants is plotted in figure 8.
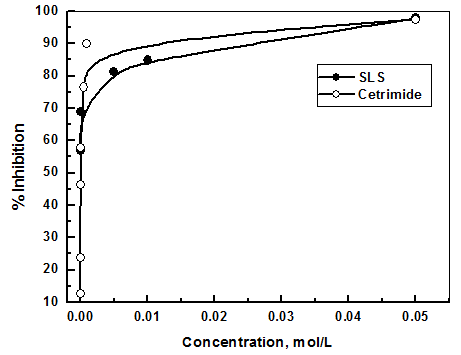 | Figure 8. Variation of the percentage inhibition of the corrosion of aluminum in 0.1 M HNO3 with concentrations of SLS and Cetrimide |
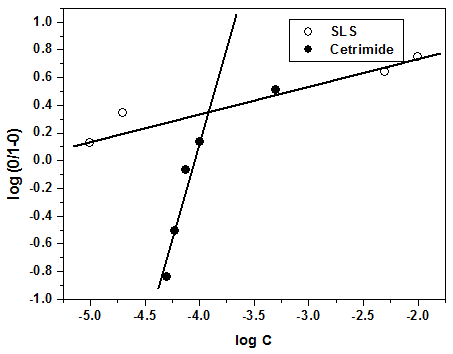 | Figure 9. Application of the Kinetic-Thermodynamic model to the results of adsorption of SLS and Cetrimide on steel surface in 0.1 M HNO3 |
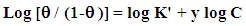 | (3) |
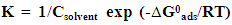 | (4) |
|
4. Conclusions
- Ÿ Both Cetrimide and SLS act as anodic corrosion inhibitors for aluminum in aqueous solutions containing nitrate ion.Ÿ Cetrimide decreases the potential of aluminum to pitting corrosion in nitric acid while SLS couldn't hinder the pitting corrosion of aluminum in nitric acid.Ÿ Tween 80 has very low efficiency toward decreasing the corrosion of aluminum in nitric acid solution.Ÿ The adsorption of surfactants onto the aluminum surface is physical in nature.Ÿ SLS and Cetrimide can solve the problems arises in the water treatment systems that caused by corrosion. Cetrimide can be used during the acid pickling process in presence of nitric acid to prevent the pittig attack that may occur.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML