-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2019; 9(1): 8-11
doi:10.5923/j.aac.20190901.02

Evaluation of Phenolic Acids in the White Wines from Slovakian “Malokarpatský” Region
Jarmila Švancarová Laštincová
Central Controlling and Testing Institute in Agriculture, Matuskova, Bratislava, Slovakia
Correspondence to: Jarmila Švancarová Laštincová, Central Controlling and Testing Institute in Agriculture, Matuskova, Bratislava, Slovakia.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The wide spread of organic compounds are presented in the white wine aromas and some of them have got fluorescence character. The main of this study was evaluate four volatile compounds present in the Slovakian protected wines from wintage year 2016. Influence of phenolic compounds in the white wine aromas is very high and are characteristic for the typical wine varieties. In this work direct estimation of vanillic acid, gallic acid, caffeic acid and p-coumaric acid was done. Identification and quantification of these compounds was performed by HPLC analysis with the fluorescence detector. In the four white wine varieties: Pálava, Traminer rose, Pinot gris and Rheinriesling with protected designation of origin “Malokarpatský” winegroving region in Slovakia there were determined identities of the different chromatographic peaks for gallic, vanillic, caffeic and para-coumaric acids. The detection limits were calculated and were 0,9-1,6μg.l-1. Detection limits were calculated as three times the standard deviation of the background.
Keywords: Phenolic acids, Fluorescence, White wine, Grape varieties
Cite this paper: Jarmila Švancarová Laštincová, Evaluation of Phenolic Acids in the White Wines from Slovakian “Malokarpatský” Region, Advances in Analytical Chemistry, Vol. 9 No. 1, 2019, pp. 8-11. doi: 10.5923/j.aac.20190901.02.
1. Introduction
- The wine is enjoyed by everyone not just because of that it contains alcohol, sugar and refreshing acids but also because contents of aromas and antioxidant components are in. Wines containing higher amounts of these biologically active substances are showing positive effects on human health (De Nisco et al. 2013). Therefore the determination of phenolic acids is needed. The techniques used in winemaking process play fundamental role in the final quality of the wines. Because of the strong influence of phenolic compounds in white wine quality, as well as the decisive role of the released odor-active volatile compounds reflecting the particular variety, climate and soil (Roujou-de-Boubee, 1999, Pasquale Crupi, et al., 2013) their detection was made in most popular Elesko Wine Park (in “Malokarpatský” i.e. “Small Carpathians Region”). We analysed Slovak wines, which were made from the most often used varieties of Vitis Vinifera L.: Traminer rose, Pálava, Pinot gris and Rheinriesling. These vine varieties are used to make white wines with high quality like wines with attribute late harvest, grape selection or berry selection which are with protected designation of origin /PDO/ in Slovakia (Laštincová et al., 2016). Protected names are prized by businesses. The labelling comes with strict regulations on production and is regarded as a quality guarantee that helps sales. The most common way to classify all these high quality variety wines is by monitoring the content of volatile aroma compounds. The aroma compounds analysis could be used for wine classification, quality control or study of sensorial properties (Lakatošová J., 2010). During maceration process it is important the interaction between thiols and phenols compounds in grape (P. Pavloušek, 2011). In the ripening there are phenolic acids which guarantied aromas and could determine the right date of harvest. Many experienced growers recognize that extending the “hang time”, in otder words, the length of the whole growing season, without getting overripeness, improves the level of aroma, colour and tannin (Ján Domin 2017). The Pálava variety was crossed from Traminer rose and Muller Thurgau in 1977 in the Czechoslovak Republic and ripe in the second part of August. The Traminer rose is in Common catalogue of varieties of vine (Vestník MPRV-SR, 2018) from 1941 and ripe from the beginning of September to the end of September. The Pinot gris togather with Rhienriesling are from registered varieties in Common catalogue most rezistent to frost and ripe in the beginning of October. The three different wineyards from the same PDO (Protected designation of origin) area “Malokarpatská” in Slovakia were included in this work. HPLC with ultraviolet detection is one of the most commonly used method, but highly effective method to determine and identify phenolic acids is HPLC with fluorescence detection.
2. Material and Methods
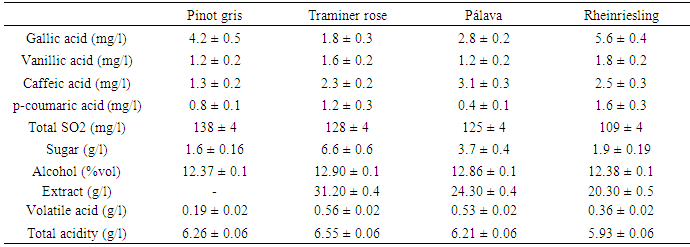
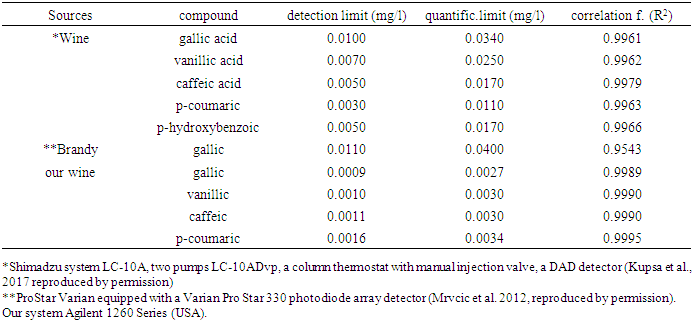
- The white wines vintage year 2016 were received from famous wine producers in Slovakia: Traminer rose and Pálava originated from Elesko a.s., Modra, SR.Pinot gris originated from the winery Topoľčianky s.r.o., Topoľčianky, SR.Rheinriesling originated from Dufrex s.r.o., Hurbanovo, SR.All wine samples were analysed during certification process in Central Controlling and Testing Institute in Agriculture in Bratislava. The standard parameters like sugar, alcohol, extract, volatile acids, total acids and total SO2 were determined according to OIV methods. The same wine samples were undergo sensorial analysis and then were analyzed to determine phenolic acids. The method described by Canas et al. (2003) and by Žiak et al. (2014) was used for measurements of concentrations of individual phenolic acids. HPLC chromatographic system Agilent 1260 Series consisting of a quaternary pump equipped with an injection valve (Rheodyne), thermostat and fluorescent detector. A personal computer running the chromathographic software and chromatographic column was LiChrospher 100 RP-18 (5 μm) LiChroCART 250-4 connected with guard column packed with the same sorbent (LiChrospher 100 RP-18 (5 μm). The temperature of separation was 40°C and mobile phase for elution was following mixtures: methanol 70 vol. /water 28 vol. / formic acid 2 vol. (A) and 98 vol. water/ 2 vol. formic acid (B). The gradient elution programme was as follows: from 0-3 min. 0% (A), from 3 min.-25 min. it was 0-40% (A), from 25 min.- 43 min. there was 40-60% (A), in the time 43 min.-55 min. there was 60% (A), from 55-60 min. there was 60-80% (A), in the time 60-65 min. there was 80% (A) and from 65-68 min. 80-0 % (A) using a flow rate 1.0 ml/ min. The total length time of analysis was 68 minutes and the regeneration was 5 minutes. Samples were filtered through a 0.45μm membrane and analyzed directly with injection of 20 μL and detected with fluorescence detector. The fluorescence detector was operated at λex = 278 nm and λem = 360 nm (gallic and vanillic acids) or λex = 278 nm and λem = 454 nm (caffeic and p-coumaric acids). The different chromatographic peaks for each phenolic acid were confirmed by comparing their spectral characteristics to standards and retention times. Chemicals were of HPLC supergradient purity. With fluorescence detection a linear calibration range was found from 5-500μg/L. Triplicate analysis of each wine variety was performed and mean value was determined. Detection limits were also calculated as 3 times the standard deviation of the background noise of each calibration graph and were 0.9-1.6μg/L for fluorescence detection. Chromatograms were recorded as you can see on Fig.1.
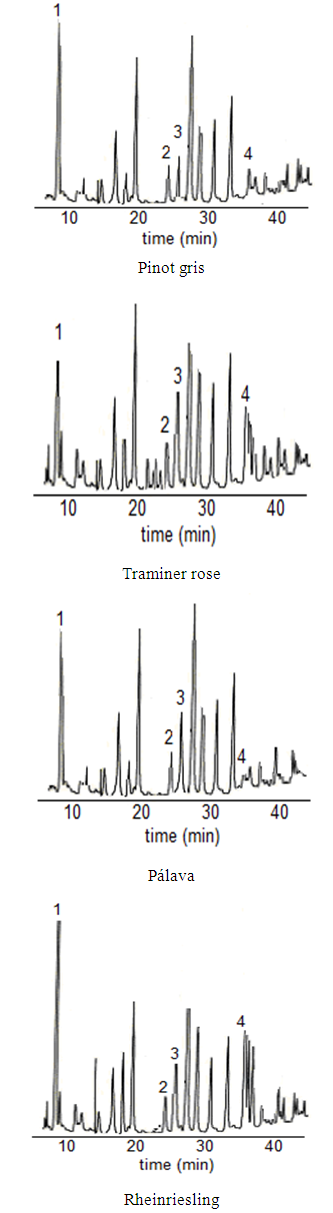 | Figure 1. Phenolics acids detected in the wine samples vintage 2016 from “Malokarpatský” region in Slovakia, (1) gallic acid, (2) vanillic acid, (3) caffeic acid, (4) p-coumaric acid |
3. Results and Discussion
- In all wine samples the folloving phenolic compounds: hydroxybenzoic acids and hydroxycinnamic acids were analysed by HPLC with fluorescence detection.Hydroxycinnamic acids are the main phenolic compounds in white wines and originate from hydroxycinnamic tartaric esters present in grapes. In grapevine, they occur above all in the pulp of berries. Their content is rather independent of the winemaking process and the most important are caffeic, p-coumaric and ferulic acids. Plant antioxidant activity is due to phenolic acid content, especially caffeic and p-coumaric. The greater antioxidant activity of caffeic acid than p-coumaric acid stems from the 3,4-position of dihydroxylation on the phenolic ring (Katalinic, 2006). Phenolics behave as antioxidants due to the reactivity of the phenol moiety (hydroxyl substituent) on the aromatic ring (Kim, 2004) and also have got fluorescent character. Caffeic acid is expected to have higher antioxidant activity because of additional conjugation in the propanoic side chain.Determined were also antioxidants that typically occur in wine like gallic and vanillic acids. These hydroxybenzoic acids found in Riesling wines from Slovakia is significant as in Germany or Czech Republic (Tomaskova et al. 2017). The chromatograms (See Fig.1.) with identification of the compounds in different wine varieties were achieved by comparing spectra and the retention times of the separated peaks with the retention times of the standards. Quantification was made by the method using calibration of standards as a reference and was based on peak area from HPLC. The different amounts of these compounds were detected in different varieties of white wines year 2016 (see Tab.1.). The varieties of white wines which ripen in October have got higher amounts of gallic and p-coumaric acid. They are higher and mainly responsible for the flavor attributes typical for Riesling (Kupsa J. et al. 2017). Total polyphenols content in different sources like spirit drinks, brandy, white wine or red wine are compared in Table 2. Most of analytical methods are based on HPLC technique with either UV or fluorescence detection (M.A.Rodríguez-Delgado et al. 2001). Varieties which ripen earlier in august-september like Pálava, have got higher content of caffeic acid. For the Traminer rose terpenes are mainly responsible for flavor, this variety ripe in September, the total acids is about 7,0g/l (Ján Domin 2017 and Goliáš et al. 2016) therefore lower concentration of gallic acid was found. In white dry wines contents of phenolic compounds ranged from 0.4-5.6 mg/l and this is in agreement with literature. In Pálava low concentration of p-coumaric acid was found, but on the other hand there was higher content of caffeic acid. In the Rheinriesling was found 1.6 mg/l of p-coumaric acid and 5.6 mg/l gallic acid, which is higher amount than in other three varieties.
|
|
4. Conclusions
- The results here show a difference in the phenolic acids concentration among the examined white wine varieties. We emphasized the need to define a common determination and take measures of many parameters in wine to reduce the risks of falsification and deception of consumers. So you can guarantee better quality wines with increased traceability and more transparency of ingredients for the consumer. Analysis of phenolic compounds in wine using the HPLC method with fluorescence detector was able to differentiate 100% of the studied phenolic acids in the white dry wines. The objective of this study was to evalute whether and which phenolic compounds in wine can serve as a source of fluorescence active compounds and to compare amounts in diferent type of wine. We elaborate further the comparison of present approach by the other authors. Exeptional credit to this analyses is that the fluorescence detector gives higher selectivity. The linear calibration range for UV detection is 0.5-500 mg/l and calibration range for fluorescence detector is 5-500 μg/l. Alcoholic beverages (wine, beer, cider, spirits), are not, at this time, subject to the labeling of additives except in cases where there is a risk of allergies, e.g. the addition of sulfites. Consumers in Europe, however, have been putting increasing pressure on the EU Commission to demand from wine producers clear and easy to understand information about calorific intake and ingredients. The polyphenols are contributing to wine aroma, taste and color and this is a relatively simple method for direct determination of phenolic acids in dry wines.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML