-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2018; 8(1): 6-9
doi:10.5923/j.aac.20180801.02

Antimicrobial Activity of Honeys from Nsukka and Ugwuaji in Enugu State, on Selected Pathogenic Bacteria Isolated from Wound
Okeke Onyeka1, Okeke M. U.2, Ezejiofor C. C.3, Ndubuisi J. O.4
1Plastic Production Unit, Scientific Equipment Development Institute, Enugu, Nigeria
2Chemistry Department, Federal College of Agriculture, Ishiagu, Nigeria
3MicrobiologyDepartment Caritas University, Enugu, Nigeria
4Applied Science Department, Federal College of Dental Technology and Therapy, Enugu, Nigeria
Correspondence to: Okeke Onyeka, Plastic Production Unit, Scientific Equipment Development Institute, Enugu, Nigeria.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Studies were carried out to determine the anti-microbial activity of honeys from Nsukka and Ugwuaji in Enugu State, on three selected pathogenic bacteria (Staphylococcus aureus, Escherichia coli and Streptococcus pyogenes) isolated from wound. The antibacterial sensitivity test was carried out using well diffusion method. The inhibitory efficiency of the honey samples on the growth of the tested organisms increased with increase in concentration from 20 to 100%. Staphylococcus aureus (3.88 – 22.61mm) was the most sensitive to the honey samples while Streptococcus pyogenes (1.26 – 13.26mm) was the less sensitive. The zone diameters of inhibitions of the organisms at different concentrations of the honey samples were found to be statistically significant. The inhibition efficiency of the honey samples on the growth of the tested organisms was found to be dependent on concentration and type of honey used and the nature of the tested organisms.
Keywords: Bacteria, Antibacterial, Activity, Honey and inhibition efficiency
Cite this paper: Okeke Onyeka, Okeke M. U., Ezejiofor C. C., Ndubuisi J. O., Antimicrobial Activity of Honeys from Nsukka and Ugwuaji in Enugu State, on Selected Pathogenic Bacteria Isolated from Wound, Advances in Analytical Chemistry, Vol. 8 No. 1, 2018, pp. 6-9. doi: 10.5923/j.aac.20180801.02.
1. Introduction
- According to the EUCD, (2001), honey is the natural sweet substance produced by Apis mellifera bees from the nectar of plants or from the secretions of living parts of plants or excretions of plant sucking insects on the living parts of plants which bees collect, transform by combining with specific substances of their own, deposit, dehydrate, store and leave in honey combs to ripen and mature.Bogdanov et al., (2004) stated that honey is the only food sweetener that can be used industrially without processing. Honey can be classified according to its origin (such as nectar or honey dews), mode of production and preservation.Honey is a concentrated aqueous solution composed of a mixture of glucose and fructose but also contains at least 22 other complex carbohydrates, various amino and organic acids, proteins, antibiotic rich inhibine, enzymes, phenol antioxidants, aroma compounds, vitamins, minerals, pigments, waxes and pollen grains (Bogdanov et al., 2007). It is viscous and acidic in nature with a pH ranging between 3.2 and 4.5. Natural honey has been used as an effective medicine around the world since ancient time. It has had a valued uses in traditional remedy for centuries. The ancient Egyptians, Assyrians, Chinese, Greeks and Romans employed honey for wounds and diseases of the gut (Bogdanov et al., 2008). Currently many researchers have reported the antibacterial activity of honey and found that natural unheated honey has some broad-spectrum antibacterial activity when tested against pathogenic and oral bacteria (Mauric et al., 2009; Adams et al., 2009). Honey is gaining acceptance as an agent for the treatment of ulcers, bed sores and other skin infections resulting from burns and wounds (Cooper et al., 2002).According the Lusby et al., (2005), the healing properties of honey can be Cooper ascribed to the fact that it offers antibacterial activity, maintains a moist wound environment that promotes healing and has a viscosity which helps to provide a protective barrier to prevent infection. They further, stated that its immune modulatory properties are relevant to wound repair.Many investigations reported that the antimicrobial activity of honey is due to phytochemical properties such as high content of reducing sugar, high viscosity, high osmotic pressure, low pH, low water activity, low protein content and presence of hydrogen peroxide (Molan and Cooper, 2002). Alnimat et al., (2012) stated that the main antibacterial activity in honey is hydrogen peroxide, which is produced by glucose-oxidase action. The level of peroxide in honey is determined also by the presence of catalase, which originates from the pollen of plants (Weston, 2000). The amount of hydrogen peroxide is affected by light, temperature and oxygen which varies according to the processing and storage conditions of the honey. Research has revealed a positive correlation between the endogenous hydrogen peroxide concentration and the inhibitory activity of bacteria growth by honey (Bizerra et al., 2002). Indeed honeys with a high concentration of hydrogen peroxide have higher antibacterial activity.In some cases, according to Libonatti et al., (2014), other antibacterial activity of honey is due entirely to the non-peroxide components such as acidity, osmolarity, flavonoids, phenolic compounds and lysozyme. Different studies have claimed that honey contains bioactive components such as lysozyme, a well-known antibacterial agent (Estrada et al., 2005).Abd-El Aal et al., (2007) showed that honey had a pronounced inhibitory effect (85.7%) on gram-negative bacteria (Pseudomonas aerugunosa, Enterobacter spp., Klebisella) in comparison to commonly used antimicrobial agents. A 100% inhibition was observed in the case of gram positive methicillin resistant Staphylococcus aureus in comparison to the use of antibiotics alone.Kwakman and Zaat, (2012) reported that the sugar content of honey is sufficient to retain antibacterial activity when diluted to approximately 20-40%. Based on extensive research on the medicinal uses of honey, its antimicrobial action on Stapylococcus aureus, Eschericha coli and Streptococcus pyogenes was investigated using honey samples from Nsukka and Ugwuaji in Enugu State, Nigeria.
2. Materials and Methods
- Sample collection: Twenty (20) honey samples each were purchased from the local beekeepers in Nsukka and Ugwuaji, Enugu state, Nigeria. The samples were stored in clean and closed polyethylene flasks at 20 – 21°C in a lightless place until analysis.Bacterial strainsStrains of Staphylococcus aureus, Eschericha coli and Streptococcus pyogenes were obtained from the Medical Department of University of Nigeria Teaching Hospital and Enugu State University Teaching Hospital, Parklane, Enugu State.Antibacterial Sensitivity TestThe antibacterial activity of honey samples from the two different locations (Nsukka and Ugwuaji) in Enugu state was tested invitro against three pathogenic bacteria (Staphylococcus aureus, Eschericha coli and Streptococcus pyogenes) using well diffusion method (Cooper et al., 2002). The test samples were prepared by diluting each in sterilized water at different dilutions (concentration), 20%, 40%, 60%, 80% and also net honey (100%).Nutrient agar plates were prepared and each plate was properly inoculated with each test organism using streaking method with the help of a sterile wire loop. Wells were made using a sterile cork borer and each well was filled with different concentrations of the honey.A distance was maintained from the edges of the plates to prevent overlapping of the inhibition zones.The plates were incubated for 24hrs at 37°C. This invitro experiment was compared with the use of a sensitivity disc (Clindamycin), which served as a control.After incubation the plates were examined and the diameter of the inhibition zones was measured in triplicate for each isolate.Statistical AnalysisThe results obtained were expressed as mean ± standard deviations and differences between means were analyzed statistically using an analysis of variance (ANOVA), SPSS version 18.0 for windows. Differences were considered significantly when p ≤ 0.05.
3. Results and Discussion
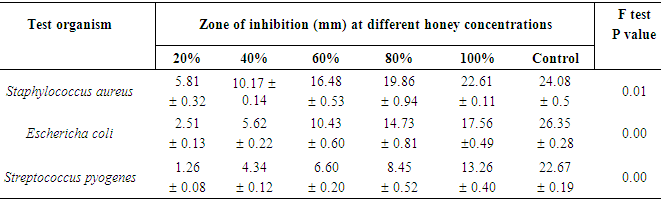
- Table 1 shows that honey samples from Nsukka at concentrations of 20, 40, 60, 80 and 100% gave 5.81, 10.17, 16.48, 19.86 and 22.61mm zone of inhibitions respectively on Staphylococcus aureus.
|
|
4. Conclusions
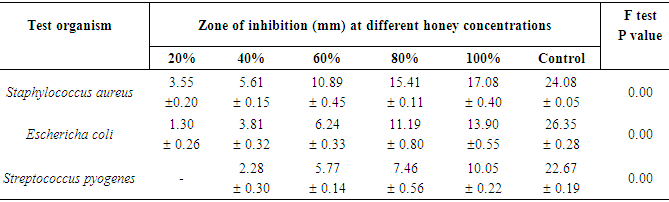
- The result of this study shows that the two different honey samples (Nsukka and Ugwuaji) inhibited the growth of the tested organisms at different concentrations. Honey samples from Nsukka gave higher inhibition efficiency on the growth of the tested bacteria than honey samples from Ugwuaji. The zone diameters of inhibitions of the organisms at different concentrations of the honey samples were found to be statistically significant.The order of sensitivity of the tested organisms to the honey samples decreased in the following order:Staphylococcus aureus > Eschericha coli > Streptococcus pyogenesFinally the bactericidal effect of the honey samples were found to be dependent on the concentration and type of the honey used and the nature of tested bacteria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML