-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2017; 7(2): 31-38
doi:10.5923/j.aac.20170702.02

Amino Acids Profile of Bee Brood, Soldier Termite, Snout Beetle Larva, Silkworm Larva and Pupa: Nutritional Implications
Olaleye Abdul Ademola1, Adubiaro Habibat Omolara2, Olatoye Rauf Abioye3
1Department of Chemistry, Faculty of Science, Federal University Dutse, Nigeria
2Department of Chemistry, Faculty of Science, Federal University Oye-Ekiti, Nigeria
3Department of Chemistry, Faculty of Science, Ekiti State University, Nigeria
Correspondence to: Olaleye Abdul Ademola, Department of Chemistry, Faculty of Science, Federal University Dutse, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

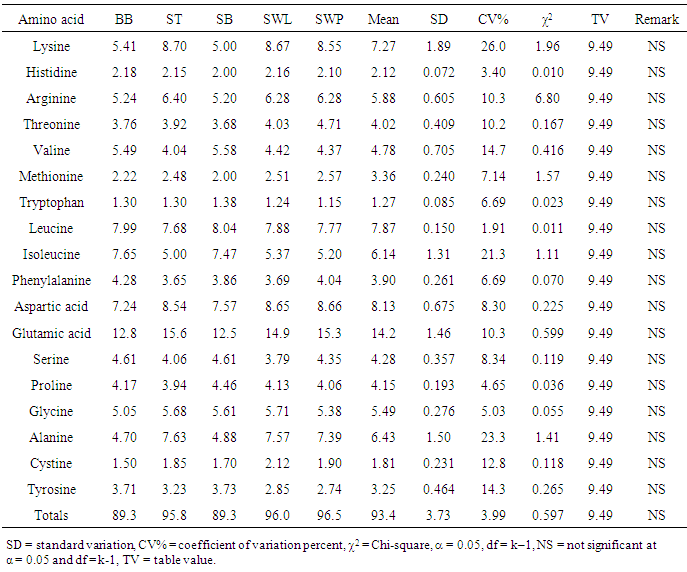
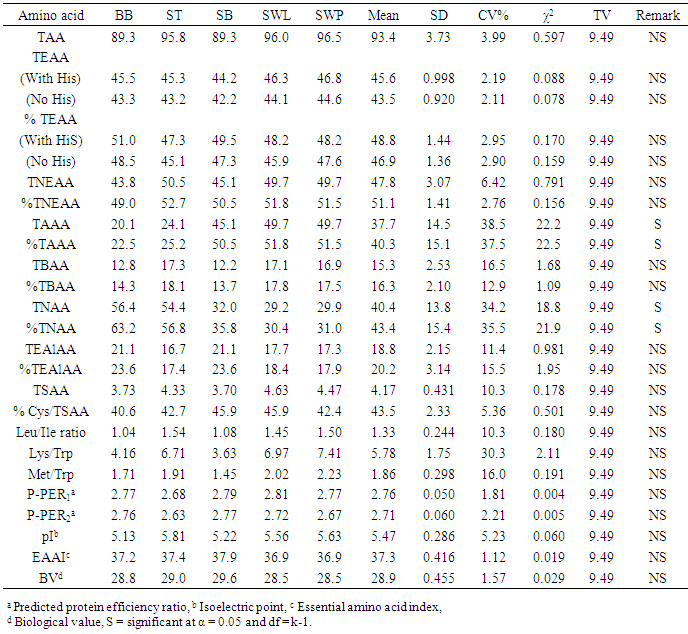
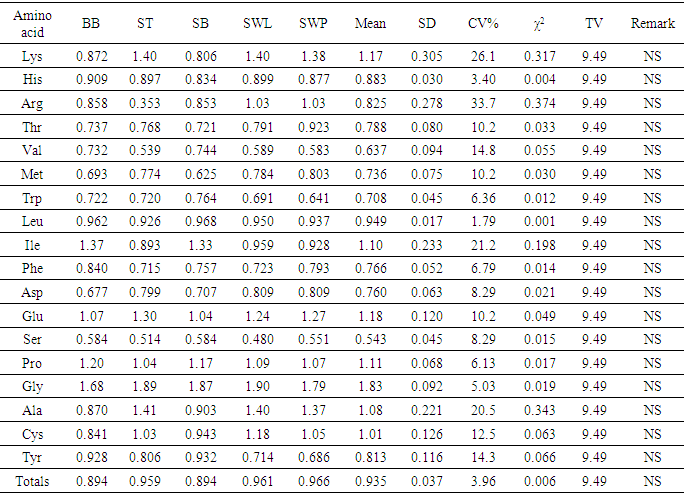
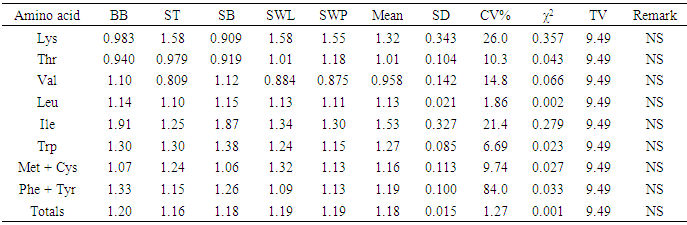
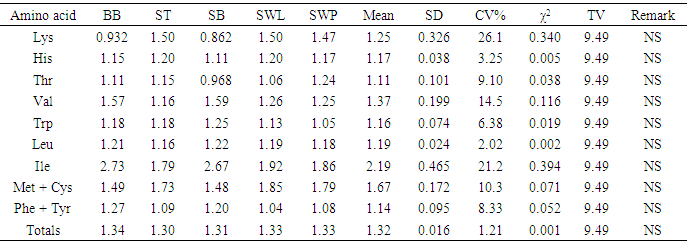
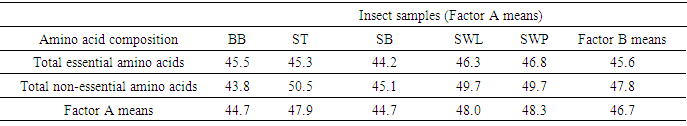
The amino acid (AA) componentst of five insect sample: bee brood (Apis mellifera, BB), soldier termite (Macrotermes bellicosus, ST), snout beetle larva (Rhynchophorus phoenicis, SB), silkworm larva (SWL) and silkworm pupa (SWP) (Anaphe infracta) were reported. Among the amino acid investigated, glutamic acid was most abundant in all the five samples with values ranging from 12.5-15.6g/100g crude protein, cp. The most concentrated essential amino acid (EAA) was leucine (7.68-8.04g/100g cp) across board whereas tryptophan (1.15-1.38g/100g cp) was the least concentrated EAA and also recorded the least values among the amino acids in all the samples. The total acidic amino acids in all the samples (20.1-49.7g/100g cp) were greater than the total basic amino acids (12.2-17.3g/100g cp). The leucine (Leu)/isoleucine (Ile) ratio values (1.04-1.54) showed that Leu > Ile across board. The predicted protein efficiency ratio (P-PER1 and P-PER2) ranges were 2.68-2.81 and 2.63-2.77 respectively. The isoelectric point (pI) range was 5.13-5.81, showing the samples to be in acidic medium of the pH range. In the amino acid scores based on whole hen’s egg glutamic acid (Glu), proline (Pro) and glycine (Gly) had scores greater than 100% in all the samples; arginine (Arg) was limiting in soldier termite whilst serine (Ser) had the least scores in others. On provisional amino acid scoring pattern, the limiting AAs were valine (Val) in ST (0.909), SWL (0.884) and SWP (0.875); Threonine (Thr) in bee brood (0.940) and Lysine (Lys) in SB (0.909). EAA scores based on pattern for pre-school child (2-5 years) showed that the samples would supply the required EAAs for this category. Generally, statistical calculations showed that no significant difference existed among the samples in most of the parameters determined.
Keywords: Edible insects, Essential amino acids, Isoelectric point
Cite this paper: Olaleye Abdul Ademola, Adubiaro Habibat Omolara, Olatoye Rauf Abioye, Amino Acids Profile of Bee Brood, Soldier Termite, Snout Beetle Larva, Silkworm Larva and Pupa: Nutritional Implications, Advances in Analytical Chemistry, Vol. 7 No. 2, 2017, pp. 31-38. doi: 10.5923/j.aac.20170702.02.
1. Introduction
- Insects are among the most diversed groups of animals that may be found in nearly all environments including the oceans. They form a class of animals within the arthropod group that have a chitinous exoskeleton, a three-part body (i.e. head, thorax and abdomen), three pairs of disjointed legs, compound eyes and a pair of antennae. There are over one thousand four hundred recorded edible insects [1]. Insects are the only winged invertebrates; cold-blooded, produced quickly and often do not have parental care [2]. A number of insects or their products were used in the past and are to a certain extent still eaten by some West African tribes, as tit-bits, or exclusively by children. Such insects are mostly those which can be collected in large numbers, e.g. locust in the gregarious phase, emerging alate termites, caterpillars and the large African cricket Brachytrypes [3]. Some insects such as enormously distended queen termite and the larvae and pupae of scarab beetles and the African silkworm, Anaphe sp. are eaten occasionally and sometimes regarded as delicacies [4]. Such consumption, besides Africa, has been practiced throughout the course of history and in all past culture including those of ancient China, Mexico, Egypt, Israel and Greece [5]. The Yukpa people of Colombia and Venezuela preferred their traditional insect foods meat as do the pedi of South Africa [6].A honey bee (Apis mellifera) is any member of the genus Apis, primarily distinguished by the production and storage of honey and the construction of perennial, colonial nest from wax. Currently, only seven species of honey bee are recognized with a total of forty-four subspecies [7]. Today’s honey bees constitute three clades: drones (males) produced from unfertilized eggs, i.e. have only a mother; workers and queens (both females) result from fertilized eggs (i.e. have both a father and a mother) [8]. Along with wasps, honey bees are the most important food insects in northern Thailand [9]. Soldier termites are defensive specialists. They have anatomical and behavioural specialisations and their sole purpose is to defend the colony. Among certain termites, soldiers may use their globular heads to block their narrow tunnels [10]. Some unique ones can spray noxious, sticky secretions containing diterpenes at their enemies [11]. Soldiers of larger termite species are consumed in the Central African Republic, the Democratic Republic of Congo, the Bolivian Republic of Venezuela and Zimbabwe [12]. They are often fried or pounded into cakes. Sometimes, for example, in Uganda, only the heads are eaten [13].Silkworm is the caterpillar of the domesticated silkmoth, Bombyx mori. It is an economically important insect, being a primary producer of silk. Silk production is an ancient practice in many parts of Asia and Europe. The worm is considered a commercially viable product. They are considered delicacies and are traditionally eaten and sold in many markets in North Eastern China. Palm weevils especially snoult weevils are highly valued as human food by people of Manipur State of North Eastern India who see insects as cheapest sources of animal protein. They are typically collected, washed and fried for consumption [14]. It is unusual to add oil because the larva exude enough oil during the frying process. Their delicious flavor is credited to their elevated fat content [14].The fact remains that people need to consume adequate calories and nutrients to overcome the problem of protein-energy malnutrition (PEM) [15]. Kent [16] attributed the wide spread malnutrition prevalent among the rural communities in Africa to inadequate and poor quality food supply. A greater number of people in such areas, due to their poverty level commonly depend on one stable food usually of carbohydrate source. Most of these insects are readily available especially in the rural areas but they are underutilized. However, many developing regions of Africa (including Nigeria), Asia, Central and South America consume many of the insects especially termites based on seasonal ubiquity either as dessert, appetizer or as protein supplement in their diet. For instance, DeFoliart, [17] stated that ‘‘the Yukpa people of Colombia and Venezuela and the Pedi people of South Africa prefer termite foods to fresh meat in some of their traditional diets. The objective of this study therefore is to reveal the amino acids composition of the commonly eaten insects and provide useful information that can further suggest their consideration as alternative sources of nutrients particularly protein.
2. Materials and Methods
- Sample collection and preparation The insect samples were obtained from farms and markets around Ekiti and neighbouring states and were later identified in the Zoology Department of Ekiti State University, Ado-Ekiti. They were screened to eliminate the defective ones, washed and rinsed with distilled water. The samples were then dried in an oven at 45°C and dry milled separately to fine powder, stored in a dry, cool place prior to use for various analyses as described below.Sample analysisAbout 1g of each sample was weighed into the extraction thimble and the fat extracted with chloroform/methanol mixture (2:1v/v) using a Soxhlet apparatus [18]. The extraction lasted 5-6 h. About 30mg of the defatted sample was weighed into glass ampoules. 7ml of 6M HCl were added and oxygen expelled by passing nitrogen gas into the sample. The glass ampoules were sealed with a Bunsen flame and put into an oven at 105 ± 5°C for 22 h. The ampoule was allowed to cool; the content was filtered to remove the humins. The filtrate was evaporated to dryness at 40°C under vacuum in a rotary evaporator. Each residue was dissolved with 5ml acetate buffer (pH 2.0) and stored in a plastic specimen bottle and kept in a deep freezer. Amino acid analysis was by ion exchange chromatography (IEC) using the Technicon Sequential Multisample (TSM) Amino Acid Analyzer (Technicon Instruments Corporation, New York). The period of analysis was 76 minutes for each sample. The net height of each peak produced by the chart recorder of the TSM (each representing an amino acid) was measured and calculated.Determination of amino acid quality parameters Determination of amino acid scores: Determination of the amino acid scores was first, based on whole hen’s egg [19]. In this method, both essential and nonessential amino acids were scored. The essential amino acid score was calculated using the provisional essential amino acid scoring pattern [20]. Amino acid score based on pre-school child essential amino acid requirement for ages 2-5 years was also calculated [21].Other determinations such as total amino acid (TAA), total essential amino acid (TEAA), total non-essential amino acid (TNEAA), total acidic amino acid (TAAA), total basic amino acid (TBAA), total aromatic amino acid (TEAlAA), e.t.c. and their percentages were made. Total sulphur amino acid (TSAA) and percentage cystine in TSAA (% Cys in TSAA) were also calculated. The Leu/Ile, Lys/Trp and Met/Trp ratios were computed.The predicted protein efficiency ratio (P-PER) was calculated using one of the equations derived by Alsmeyer et al. [22], i.e.
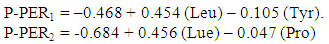 The isoelectric point (pI) was calculated using the equation of the form Olaofe and Akintayo [23]:
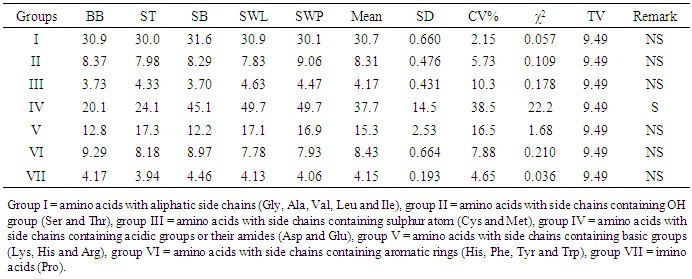
The isoelectric point (pI) was calculated using the equation of the form Olaofe and Akintayo [23]: 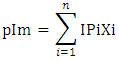 Where pIm is the isoelectric point of the mixture of amino acids, IPi is the isoelectric point of the ith amino acid in the mixture and Xi is the mass or mole fraction of the ith amino acid in the mixture [24]. The essential amino acid index (EAAI) and biological value (BV) were calculated by the method of Oser [25]. BV = 1.09 (EAAI) – 11.73.The various amino acid groups into classes I-VII [26] were also calculated.Thirty (30) insects each of bee brood and soldier termite; ten insects each of snout beetle, silkworm larva and silkworm pupa were blended together for the analyses and each analysis was carried out in duplicate.
Where pIm is the isoelectric point of the mixture of amino acids, IPi is the isoelectric point of the ith amino acid in the mixture and Xi is the mass or mole fraction of the ith amino acid in the mixture [24]. The essential amino acid index (EAAI) and biological value (BV) were calculated by the method of Oser [25]. BV = 1.09 (EAAI) – 11.73.The various amino acid groups into classes I-VII [26] were also calculated.Thirty (30) insects each of bee brood and soldier termite; ten insects each of snout beetle, silkworm larva and silkworm pupa were blended together for the analyses and each analysis was carried out in duplicate.3. Results and Discussion
- The amino acids composition of the five insect samples (dry weight): bee brood (Apis mellifera), soldier termite (Macrotermes bellicosus), snout beetle larva (Rhynchophorus phoenicis), silkworm larva and pupa (Anaphe infracta) was presented in Table 1. Among the amino acids analyzed, Glu had the highest concentration in all the samples with the values ranging between 12.5 g/100g cp in snout beetle larva and 15.6 g/100g cp in soldier termite. Tryptophan (Trp) recorded the lowest concentration (1.15-1.38g/100g cp) among the amino acids in all the samples. The most concentrated essential amino acid was lysine in soldier termite (8.70g/100g cp), silkworm larva and pupa (8.67g/100g cp and8.55g/100g cp respectively). The highest EAA was leucine (Leu) in bee brood (7.99g/100g cp) and snout beetle larva (8.04g/100g cp). Arg (5.20-6.40g/100g cp) and Ile (5.00-7.65g/100g cp) were also high in abundance. The presence of substantial amount of arginine in diets enhances Ca2+ absorption, but under most physiological circumstances, this is of little consequence [27]. Histidine (His) ranged between 2.00-2.218 g/100g cp. His is a precursor of histamine, a substance normally present in small amounts in cells [28]. Children do not grow if His is absent from their diet, but adults can probably synthesize enough for their daily needs. Threonine (Thr) in the insect samples ranged between 3.68-4.71 g/100g cp with the highest concentration recorded in silkworm pupa (4.71g/100g cp). The concentration of methionine (Met) ranged between 2.00-2.57 g/100g cp. Met contains sulphur in the thioether linkage. Met is needed for the synthesis of other important substances, including choline. It is worthy of note that the FAO/WHO/UNU [21] standards for pre-school children (2-5 years) are (g/100g cp): Leu (6.6), Ile (2.8), Lys (5.8), Met + Cys (2.5) and His (1.9). Hence, the insects in this study would be able to provide the required or even more than the required Leu, Ile, Met + Cys and His. Three samples (g/100g cp): soldier termite (8.70), silkworm larva (8.67) and silkworm pupa (8.55) would provide more than the requirements. Generally, comparison of results among the samples showed no significant variation as it was evident in the levels of coefficient of variation percent (CV%).
|
|
|
|
|
|
|
4. Conclusions
- The results of the study showed that the insects samples investigated are rich in amino acids especially Leu, Ile, Asp and Glu. The concentrations total non-essential amino acids were fairly above those of essential amino acids. The concentrations of leucine were generally higher than those of isoleucine. Dietary excess of leucine could be counteracted by increasing the intake of niacin or tryptophan or by supplementation with isoleucine [40]. The P-PER values in the samples were low and therefore should be supplemented with foods of considerably high P-PER values. The amino acid scores based on whole hen’s egg amino acid, FAO/WHO [20] standard and pre-school child requirements showed Ser, Arg, Val, Thr and Lys as being limiting. Summarily, the samples are good sources of amino acids and would be useful in food fortification.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML