-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2017; 7(2): 23-30
doi:10.5923/j.aac.20170702.01

Triton X-100 and Sodium Lauryl Sulfate Surfactants as Environmentally Friendly Corrosion Inhibitors for Steel in 0.5 M H2SO4
O. A. Abdullatef
Faculty of Pharmacy, Pharos University, Smoha, Alexandria, Egypt
Correspondence to: O. A. Abdullatef, Faculty of Pharmacy, Pharos University, Smoha, Alexandria, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effect of anionic surfactant Sodium Lauryl Sulfate and neutral surfactant Triton X-100, as environmentally friendly corrosion inhibitors for carbon steel in 0.5 M H2SO4, has been tested. The inhibition efficiency has been determined by weight loss and impedance measurements. Adsorption of Sodium Lauryl Sulfate and Triton X-100 on carbon steel in 0.5 M H2SO4 obeyed Langmuir adsorption isotherm. Mechanism of adsorption has been studied and explained. Effect of TX 100 on the kinetics of the dissolution of steel in 0.5 M H2SO4 was studied. The activation parameters Ea, ∆H* and ∆S* were calculated and explained.
Keywords: Surfactants, Acid Corrosion, Adsorption, Inhibition, Weight loss, Impedance
Cite this paper: O. A. Abdullatef, Triton X-100 and Sodium Lauryl Sulfate Surfactants as Environmentally Friendly Corrosion Inhibitors for Steel in 0.5 M H2SO4, Advances in Analytical Chemistry, Vol. 7 No. 2, 2017, pp. 23-30. doi: 10.5923/j.aac.20170702.01.
Article Outline
1. Introduction
- Metal corrosion is electrochemical interaction between the metal and the surrounding environment leading to degradation of the metal properties. Loss of metal by corrosion is not only waste of the metal, but also waste of energy, water, and human effort that are used to fabricate the metal structures. The main reasons for the importance of corrosion are: safety, economics and conservation. Corrosion can affect the safety of operating equipment by causing failure to pressure vessels, boilers, metallic containers for toxic chemicals and airplane components. Also, it is a critical issue in the design of equipment for nuclear power plants and for disposal of nuclear wastes. Direct economic losses include the costs of replacing corroded structures and machine or their components, such as condenser tubes, pipelines and the extra cost of using corrosion - resistant metals and alloys instead of carbon steel where the latter has suitable mechanical properties but not sufficient corrosion resistance. Indirect economic losses includes; Shutdown of the unit and loss of product, loss of efficiency because of diminished heat transfer through accumulated corrosion products, Contamination of product, Overdesign in which equipment is often designed many times heavier than normal operating pressures. Losses would be cut at least in half because less power would be required to operate [1, 2]. Although there are a large number of organic compounds used as corrosion inhibitors, there was a general trend for many decades to find new trends of corrosion inhibitors for metals. Nowadays, there are restrictions to the use of ordinary corrosion inhibitors like chromates, nitrites, molybdates, and all organic inhibitors due to their chemical hazards. This has prompted the search for eco-friendly corrosion inhibitors for metals. Thus, there has been increased interest in employing environmentally benign corrosion inhibitors for metals in acid solution for sustainable development. Plants extracts are one of these sources. Plant extracts serve as environmentally friendly corrosion inhibitor materials [3, 4]. Among these newly discovered inhibitors are surfactants. Although, they are chemical compounds but they are environmentally acceptable, inexpensive, readily available and renewable source of materials [5-7]. Surfactants are widely used in our daily life and in industrial applications [8]. Surfactants nowadays find its role as corrosion inhibitors [9, 10]. The advantages of using surfactants as corrosion inhibitors are its low toxicity, low price, and easy production [11]. The mechanism of action of surfactants as corrosion inhibitor is adsorption on the metal surface in such a way that the polar or ionic part (hydrophilic) attaches the metal surface while the non-polar part (hydrophobic) extends in the solution [12]. It was also reported that the adsorption behavior depends on the structure of the surfactant [13]. Leema Rose et al. [14] studied the effect of Sodium Dodecyl Sulphate and Calcium Propionate in biocidal activity and corrosion inhibition of mild Steel immersed in an aqueous acidic solution using weight loss and biocidal efficiency method. The results showed that addition of SDS increases the inhibition efficiency and a protected film is formed. El-Tabei el al. [15] studied the synthesis and inhibition effect of a novel tri-cationic surfactant on mild steel corrosion in 0.5 M H2SO4 solution using potentiodynamic polarization, electrochemical impedance spectroscopy and weight loss techniques. The three techniques showed that prepared Tricationic surfactant acted as mixed-type inhibitor for mild steel in 0.5 M H2SO4 and the corrosion inhibition increased with increasing the inhibitor concentration and temperature. In our previous work, [16, 17] we studied the effect of the cationic surfactant Cetyl trimethyl ammonium bromide on the electrochemical behavior of steel in 0.5 M H2SO4 solution and the effect of Tween 80 neutral surfactant on aluminum in 0.5 M HCl.The aim of this study is to focus on the effect of surfactants: Sodium Louryl Sulphate (SLS), TritonX-100 on the electrochemical behavior of mild steel in sulfuric acid and to study the adsorption mechanism of those surfactants on the metal surfaces using electrochemical impedance spectroscopy (EIS) and weight loss techniques in this medium.
2. Materials and Methods
2.1. Electrochemical Measurements
- A frequency response analyzer potentiostat (ACM 604) was used to carry out the electrochemical impedance and polarization measurements. The frequency range for EIS measurements was 0.1 x 104 to 0.01 Hz with applied potential signal amplitude of 10 mV around the rest potential. A three electrode mode cell contains an auxiliary graphite electrode and saturated calomel reference electrode was used. The working electrode was fabricated in cylindrical form. Steel was encapsulated in epoxy resin in such a way that only one surface was left uncovered. The working electrode has the chemical composition (% wt) Fe 96.86; C 0.21; S 0.04; Mn 2.5; P 0.04; Si 0.35. The exposed area (0.28 cm2) sample was wet hand-polished using different grade emery papers of variable grades starting with a coarse one and proceeding in steps to the finest (1000) grade. The sample was then washed thoroughly with double-distilled water and finally dried by absolute ethanol just before immersion in the solution. Each experiment was carried out with newly polished electrode. Before EIS measurements, the working electrode was introduced into the test solution and left for 20 min at the open circuit potential. All the measurements were done at 30.0 ± 0.1°C in solutions open to the atmosphere under unstirred conditions. To test the reliability and reproducibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.2. Weight Loss Measurements
- Rectangular specimens of mild steel with dimensions (2.0 cm x 5.0 cm x 0.05 cm) were used during weight loss measurements. The weight loss coupons were polished, cleaned and weighted, then suspended in beakers containing 100 ml acidic solution with and without different concentrations of surfactant. After definite time, the coupons were removed from the solution, washed with distilled water, ethanol and then dried by acetone and reweighed. The weight loss was then determined (gm/cm2), the experiment was then repeated for different time intervals up to 6 hours. To test the reliability and responsibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.3. Solution Preparation
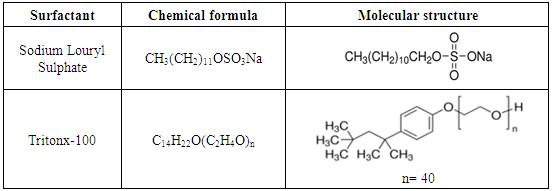
- The test solutions were prepared from analytical grade reagents and distilled water. 98% H2SO4 was purchased from Aldrich chemicals. Stock solution, of 3 M of H2SO4 and 0.02 M of surfactant were used to prepare the test solution. Prior each experiment, certain volume of 3 M of H2SO4 is added to an appropriate volume of 0.1 M surfactant solution and double distilled water to obtain a solution of 0.5 M H2SO4 and the required concentration of the surfactant. Triton X-100 (TX 100) and Sodium Lauryl Sulphate (SLS) were obtained from Alpha Chemica, their molecular structures are given in Table 1.
|
3. Results and Discussion
- The effect of neutral surfactant Triton X-100 (TX 100) and anionic surfactant Sodium Lauryl Sulfate (SLS) on the electrochemical behavior of steel in 0.5 M H2SO4, was studied using weight loss and electrochemical impedance spectroscopy (EIS) techniques.
3.1. Mass Loss Measurements
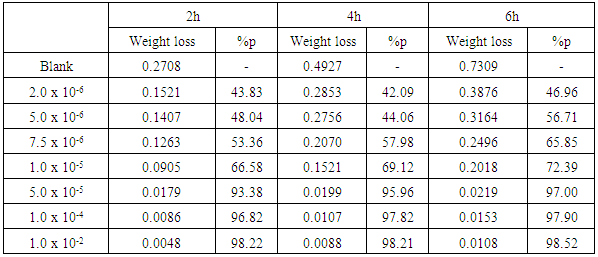
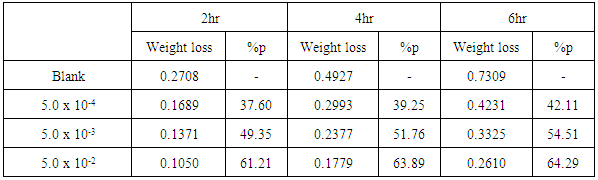
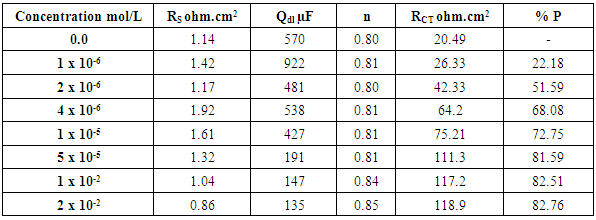
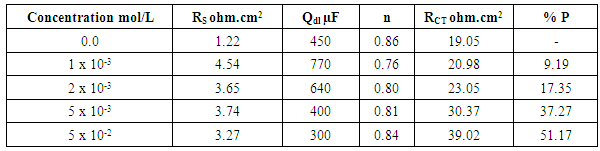
- Tables 2 and 3 present the mass loss results obtained for the corrosion of steel in 0.5 M H2SO4 in absence and presence of different concentrations of TX 100 and SLS surfactants at different immersion times.
|
|
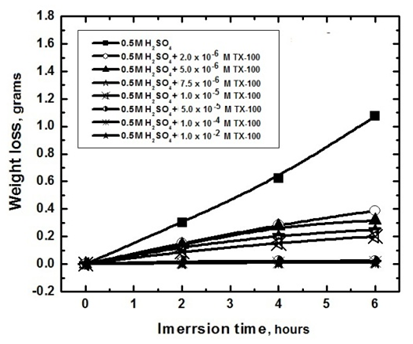 | Figure 1. Mass loss measurements of steel immersed in 0.5 M H2SO4 in presence and absence of different concentrations of Triton X 100 surfactant against different immersion times (at 30°C) |
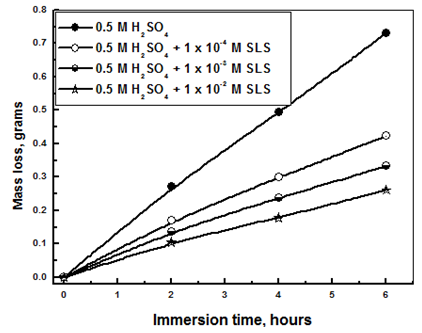 | Figure 2. Mass loss measurements of steel immersed in 0.5 M H2SO4 in presence and absence of different concentrations of SLS surfactant against different immersion times (at 30°C) |
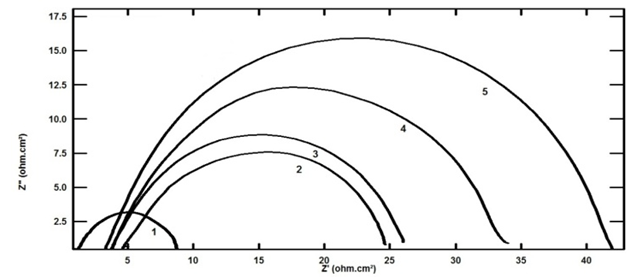
3.2. Electrochemical Impedance Spectroscopy Measurements (EIS)
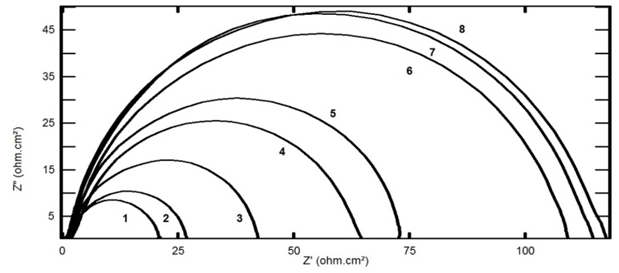
- Figures 3 and 4 represent Nyquist plots for steel immersed in 0.5 M H2SO4 in presence and absence of different concentrations of TX 100 and SLS respectively.
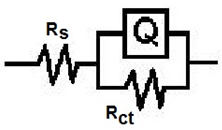 | Figure 5. Equivalent circuit |
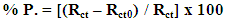 | (1) |
|
|
3.3. Application of Langmuir Adsorption Isotherm
- It is known that the surfactant molecules have a tendency to associate at interfaces and in solution to form aggregates [5]. Adsorption of the surfactant molecules on to the metal surface, which is the primary action of the surfactant functional group, was found to be responsible for the corrosion inhibition of the metal and is related to its capability to aggregate to form micelles. The variation of the percentage inhibition, which was calculated from the mass loss measurements, with different concentrations of both SLS and TX 100 surfactants is plotted in figure 6.
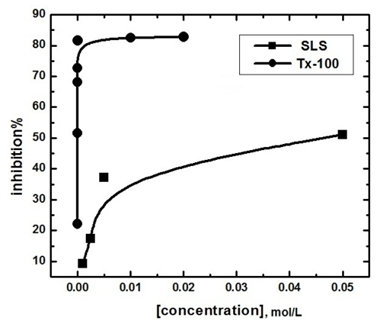 | Figure 6. Variation of the percentage inhibition of the corrosion of steel in 0.5 M H2SO4 with concentrations of SLS and TX 100 |
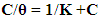 | (2) |
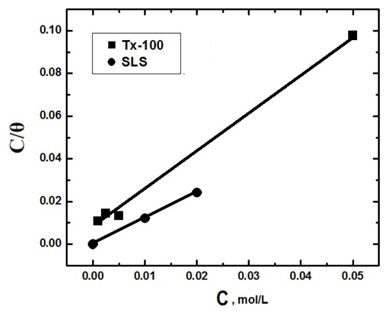 | Figure 7. Application of Langmuir adsorption isotherm to the results of adsorption of SLS and TX 100 on steel surface in 0.5 M H2SO4 |
 | (3) |
|
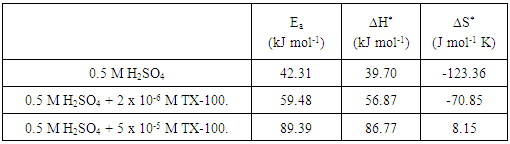
3.4. Effect of TX 100 on the Kinetics of the Dissolution of Steel in 0.5 M H2SO4
- The effect of TX 100 on the kinetics of the dissolution of steel in 0.5 M H2SO4 was studied by comparing the activation parameters of the dissolution reaction of steel in the free acid in absence and in presence of two different concentrations of TX 100 surfactant. The results obtained are applied in the linear square fit of ln W and ln (W/T) versus (1/T) according to Arrhenius and Transition State equations [22] as following:Arrhenius equation:
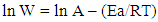 | (5) |
 | (6) |
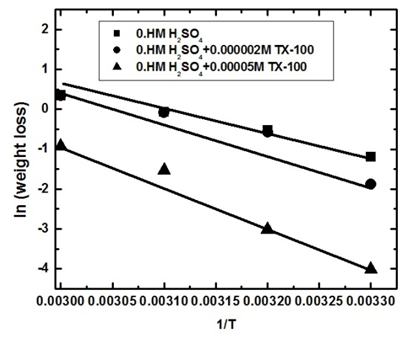 | Figure 8a. Linear fit for (ln W) data vs. (1/T) for steel dissolution in 0.5 M H2SO4 solutions in the absence and presence of 2 x 10-6 and 5 x 10-5 M TX 100 |
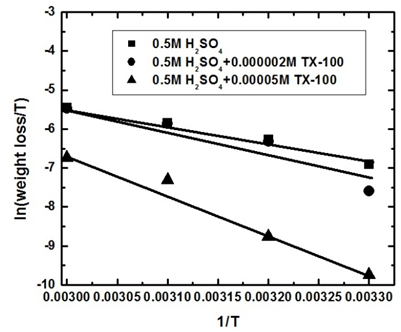 | Figure 8b. Linear fit for (ln W/T) data vs. (1/T) for steel dissolution in 0.5 M H2SO4 solutions in the absence and presence of 2 x 10-6 and 5 x 10-5 M TX 100 |
|
3.5. Mechanism of Adsorption of Surfactants
- The adsorption of surfactant onto the metal surface can be explained by considering the changes that could happen to the structure of the double layer formed at the metal/solution interface. Characteristics of this region is the existence of a specific structure of particles and the presence of an electric field of significant intensity across it which is caused by the separation of charges that are in contact. The surface phase can be considered as a parallel plate condenser, with one plate on the center of the ions that have been brought to the electrode. At the distance of their closest approach to it, and with the second plate at the metal surface, between the two plates and acting as a dielectric are oriented water molecules. This structure is known as the electric double layer. Thermal motion of the positive ions in the solution makes the condenser plate on the electrolyte side of the interface diffuse. This condition justifies the potential gradient from the metal surface through the bulk of the solution. The picture is further complicated by the presence of ions in the surface layer of the electrode in addition to those that are present due to the force of attraction or repulsion between electric charges. Such electrode surface layer ions are said to be specifically adsorbed on the electrode surface [25]. Adsorption of surfactants on the metal surface can change their interfacial properties such as hydrophobicity, surface charge and corrosion inhibition. Generally, adsorption of the surfactant on the metal surface is controlled by covalent bonding, electrostatic interaction, hydrogen bonding, non-polar interaction between the adsorbed species, lateral association interaction, solvation and desolvation. The overall adsorption process is a mixture of all these forces [26].The adsorption of surfactants at the solid–liquid interface is affected by a number of factors such as: i) the nature and the surface charge of the metal (whether it contains highly charged sites or essentially nonpolar groupings); ii) the chemical structure of the surfactant (whether it is ionic or nonionic, the hydrophobic group is long or short, aliphatic or aromatic, straight chain or branched); iii) the nature of the electrolyte [27]. There are a number of mechanisms by which surfactants molecules may adsorb onto metal surface from aqueous solution [28-30]. It is reported that [31] the adsorption of ionic surfactants on oppositely charged metal surface can be explained by considering that, at low surfactant concentrations, the adsorption occur by electrostatic interaction between the charged ionic surfactant and the oppositely charged solid surface. After then, the surfactant molecules starts to aggregates on the metal surface due to lateral interactions between the hydrophobic chains which enhances the electrostatic interaction between the charged surfactant and the metal surface. When the solid surface is electrically neutralized by the adsorbed surfactant molecules, the electrostatic forces is no longer play the important role and adsorption takes place because of the lateral interaction only. When the concentration of surfactant reaches the critical micelle concentration, the surfactant activity becomes constant and any increase in concentration leads only to micellization in solution. In the last step the surfactant molecules adsorb with a reverse orientation in which the head ionic groups faced the solution resulting in a decrease in the hydrophobicity of the particles in this region which is the case in presence of SLS. The adsorption of SLS on the steel surface can be explained by considering the ion pairing which involves the negative (hydrophilic parts) ions of surfactant adsorption from solution onto oppositely charged sites (positive charge) on the metal surface unoccupied by counter ions resulting in strong interaction between the metal and the surfactant anions. In a previous work, A. Kumar studied the effect of SLS surfactant as corrosion inhibitor for steel in hydrochloric acid by using mass loss and polarization techniques. The results obtained are in good agreement with the current results indicating that SLS adsorbed on the steel surface in acidic solution regardless the type of anionic species present in solution [32].The adsorption of cationic surfactant Cetrimide on steel surface, which is positively charged, in 0.5 M H2SO4 were explained in our previous work by assuming the important role played by the counter ion Br- during the adsorption process. The driving force for the adsorption of cationic surfactant Cetrimide onto the steel surface is the intermediate bridge formed by Br- ion between the negative ends of the bromide-steel dipoles oriented towards the solution setting up an additional potential difference between the steel surface and the bulk solution. This will shift the potential of zero charge positively making the charge on the steel surface negative and enhances the adsorption of the positively charged surfactant molecules by formation of ionic bonds. The high hardness of bromide ions and cationic surfactant increase the tendency of electrostatic adsorption which in turn increase the inhibition efficiency [33].The adsorption of the neutral surfactant TX-100 on the steel surface can be explained by considering one or all the following proposed mechanisms: Adsorption by polarization of π – electrons which takes place by the electron-rich aromatic nuclei in its chain and their attraction to the solid metal which has strongly positive sites; adsorption by dispersion forces which takes place via London–van der Waals dispersion forces between metal and the large number of ethylene oxide units in the TX 100 surfactant chain, where by this mechanism adsorption of the surfactant increases with an increase in its molecular weight; hydrophobic bonding which takes place when the attraction between hydrophobic groups of the surfactant molecules due to their tendency to escape from an aqueous environment becomes large enough to permit them to adsorb onto the metal by aggregating their chains.
4. Conclusions
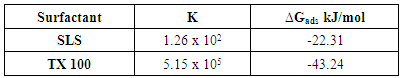
- The results showed that both TX-100 and SLS can be used as environmentally friendly corrosion inhibitors for steel in 0.5 M H2SO4. TX-100 has very high efficiency for protection of steel in 0.5 M H2SO4 as it has 97% inhibition efficiency at very low concentration 5 x 10-5 M. Langumir adsorption isotherm was applied to the experimental data. The results showed that Langumir fit the results. ∆Gads was calculated and found to be -22.1 kJ/mol for SLS indicating physical adsorption and -43.2 kJ/mol for TX-100 indicating chemical adsorption. The activation parameters were calculated by applying Arrhenius equation. Ea, ∆H* in presence of the surfactants were found to be higher than those of the free acid solution indicating that the adsorption of the surfactants at the steel/solution interface increases the energy barrier of the dissolution reaction of steel. ∆S* has negative values in the absence of surfactant indicates that the activated complex represents association rather than dissociation step. The values of ∆S* in presence of the surfactants are less negative or (more positive), meaning that the entropy increases in presence of surfactants which is the driving force for the adsorption of the surfactants on the steel surface.
ACKNOWLEDGEMENTS
- The author acknowledges the staff members of corrosion laboratory, Faculty of Science, Alexandria for giving the opportunity for doing the research in the laboratory.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML