-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2017; 7(1): 7-12
doi:10.5923/j.aac.20170701.02

Assessment of Heavy Metals in the Blood and Some Selected Entrails of Cows, Goat and Pigs Slaughtered at Wurukum Abattoir, Makurdi-Nigeria
Simon Terver Ubwa1, Rose Ejiga1, Patrice-Anthony Chudi Okoye1, Qrisstuberg Msughter Amua2
1Department of Chemistry, Benue State University, Makurdi, Nigeria
2Department of Applied Chemistry, Federal University, Dutsin-Ma, Nigeria
Correspondence to: Simon Terver Ubwa, Department of Chemistry, Benue State University, Makurdi, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study determined heavy metal levels in selected tissues and organs of these animals to sensitize the people on the danger/safety of the consumption of their meat. A cross-sectional study design with laboratory analysis component was adopted. Atomic Absorption spectrometer, buck scientific 2010 model was used to assess the residual level of some heavy metals; Cd, Cr, Ni and Pb in the blood and some selected entrails (liver, kidney, chitterlings and stomach) of cow, native goat, non native goat and pig slaughtered at Wurukum abattoir. Mean levels of residual Cr (mg/L) in the blood of the studied animals ranged from 0.07 to 0.39, 0.07 to 0.33, 0.03 to 0.20 and 0.03 to 0.19 respectively for the list above. Most of these values were found to be above the 0.1 mg/L permissible limit set by FAO/WHO for Cr in the meat of livestock animals. Similar trends were observed with the residual heavy metal levels in the entrails of cow where the Cd and Cr levels (mg/L) ranged from 0.01 to 0.03 and 0.27 to 0.57 respectively in the liver, in the kidney, their levels (mg/L) ranged from 0.03 to 0.04 and 0.15 to 0.35 respectively, while in the chitterlings, the Cd level was 0.03 and Cr ranged from 0.19 to 0.32, also in the tripe, the Cd concentration ranged from 0.20 to 0.36. In the entrails of native goat the residual level of Cd was 0.01 and Cr level ranged from 0.04 to 0.26 in the liver; the residual level of Cd and Cr ranged are from 0.01 to 0.04 and 0.06 to 0.1 respectively in the kidney and in the chitterlings, (0.01 to 0.02 and 0.29 to 0.39) mg/L respectively; in the paunch the Cd residual level was 0.01 while Cr ranged from 0.09 to 0.12 mg/L. In the entrails of non native goat: the residual level of Cd and Cr residual level ranged from 0.02 to 0.31 and 0.11 to 0.35 respectively in the liver; in the kidney it was (0.22 to 0.23 and 0.19 to 0.40) mg/L respectively; chitterlings, Cr residual level (0.13 to 0.47) mg/L; while in the paunch (0.01 to 0.04 and 0.22 to 0.30) mg/L Cd and Cr respectively. In the entrails of pig: the residual levels of Cd was 0.02 and Cr ranged from 0.19 to 0.29 respectively in the liver; Cd residual level was 0.06 and Cr level (0.11 to 0.33) in the kidney; while in chitterlings and maw, Cr level ranged from 0.10 to 0.19, and from 0.17 to 0.26 mg/L respectively. Cr residues were higher in all of the selected entrails of the animals under study than the 0.1 mg/kg permissible limit set by FAO/WHO for Cr in the meat of livestock animals while Cd residues in all the selected entrails of the various animals collected in this study were within the 0.5 mg/kg permissible limit.
Keywords: Heavy metals, Blood, Entrails, Abattoir
Cite this paper: Simon Terver Ubwa, Rose Ejiga, Patrice-Anthony Chudi Okoye, Qrisstuberg Msughter Amua, Assessment of Heavy Metals in the Blood and Some Selected Entrails of Cows, Goat and Pigs Slaughtered at Wurukum Abattoir, Makurdi-Nigeria, Advances in Analytical Chemistry, Vol. 7 No. 1, 2017, pp. 7-12. doi: 10.5923/j.aac.20170701.02.
Article Outline
1. Introduction
- The consumption of meat is of immense benefit as it provides a substantially large amount of bioavailable micronutrients to man [1]. Being one of the best sources of protein, it serves to improve the overall health and well-being of the body through the repair and building of body tissues as well as the production of antibodies [2, 3]. Meat is also a vital source of vitamins which promote good vision, develop stronger bones and support the central nervous system thus boosting mental health [4, 5]. It also contains trace elements such as zinc, magnesium, iron and selenium which play various roles in human health [5]. Some meat products contain high amounts of healthy unsaturated omega-3 fatty acids (which are polyunsaturated fats) that have a positive impact on cardiovascular health [6] and help in lowering cholesterol, blood pressure and triglyceride levels [7].Despite the underscored nutritional value of meat and meat products, its quality in some cases may be compromised by chemical contaminants. Some of these chemical contaminants are ubiquitous as a result of anthropogenic activities that mobilize them into the environment. These may make the contaminants bound to become bioavailable to animals which are the source of the meat produced through the food chain. Heavy metals are among these chemical contaminants that are prevalent in our environment. They are widely distributed as a result of leaching into the environment by erosion, industrial and agricultural processes. They also exist naturally in soils at low concentrations. In high concentrations they are a very important group of environmental toxicants since they are potent metabolic poison to humans, animals, fish and plants [8]. Heavy metal pollution in rural areas is due to disposal of effluents and sewage sludge which causes problem for grazing animals because they depose heavy metals on pastures, grasses or forages [9].Reports on heavy metal contamination of meat and other edible tissues including blood is a matter of great concern for food safety. These metals are toxic in nature and even at relatively low concentrations can cause public health hazard [10]. Miranda et al., reported high levels of some toxic and trace metals in calves and kids from a polluted area of Northern Spain [11] even as Jukna, et al., reported moderate levels of heavy metals in the viscera and muscles of Lithuanian cattle [12]. Furthermore high concentrations of some heavy metals in cattle reared in the vicinity of a metallurgic industry have also been reported [13]. Others include report of low copper and mercury levels in local Jordanian and imported goat and pig meat and organs [14] and low cadmium levels in poultry meat [15]. Similarly, Okoye et al., in his studies on blood from cows grazed in open fields in Nigeria reported high levels of cadmium, and lead [16]. It is worthy of note that heavy metals tend to bioaccumulate in different parts of the body but particularly in vital organs and tissues such as liver, kidneys, blood, stomach and intestines [17]. This same organs are preferred to the flesh due to the concentration of essential nutrients in them [1] as a result, they are mostly considered as a delicacy in Nigeria and thus prepared in various forms (“kayan chiki” or “Ngwo Ngwo” –in Huasa and Igbo languages) for consumption especially after a long day work in relaxation centres and restaurants. However, little information on the level of heavy metal accumulation in the entrails and blood of the animals; whose meat is mostly consumed in Nigeria; including the study area. It is against this background that the present study was carried out to assess the levels of these toxicants in the blood and entrails of slaughtered animals.
2. Materials and Methods
2.1. Sample Collection and Preparation
- Through vein puncture, fresh blood samples each from three (3) cows, six (6) goats (3 native and 3 non native) and three (3) pigs were collected in 25 mL clean sterilized metal- free collection tubes/ plastic bottles with gentle handling to prevent hemolysis, and kept frozen at 4°C until the time of extraction and analysis of the heavy metals to prevent platelet disintegration.Entrails samples were collected from four different types of each of the randomly selected animals: cow, goat (native and non-native) and pig from Wurukum abattoir in Makurdi. From each set of animals, selected entrails samples consisting of liver, kidney, chitterlings and stomach were collected. In this way, a total of forty eight (48) samples (24 from goats, 12 from cows and 12 from pigs) were collected. The samples were randomly collected from animals immediately after stunning. Proper identification was carried out to ensure that all tissues of the same sample were from the same animal. For each sample, 10 g was collected in small dark labeled polythene bags, which were sealed with a rubber band and stored in ice chest of about 4°C and immediately transported to the laboratory on the same day. Prior to meat collection, visible fat and connective tissues were excised. The samples were kept frozen at 4°C until the time of extraction and analysis of the samples. In the laboratory, the collect samples were cut to small sizeable pieces using clean stainless steel knife and oven dried at 105°C for about 3 hours continuously until constant weight was obtained. After drying, the samples were then subjected to acid digestion.
2.2. Digestion and Extraction of Blood and meat Samples
- The wet digestion procedure was used [18, 19]. For trace and heavy metals analysis, 0.5 mL of the blood sample was predigested with 10 mL 1:1 concentrated HNO3 and HClO4 acids on a hot plate at 135°C until the liquor had finished undergoing oxidation. Thereafter, 5 mL H2O2 was added and temperature was maintained at 135°C for 1 hour until the liquor got completely digested and showed a clear colour. For entrails samples, 2.0 g of each sample were placed in a digestion tube and predigested with 10 mL concentrated 1:1 HNO3 and HClO4 acids. Digestion tubes with contents were placed on a hot plate at 135°C until the liquor had finished undergoing oxidation [20]. Thereafter, 5 mL H2O2 was added, then 100 mL deionized water was added and then boiled for 11/2 hours. The temperature was continuously maintained at 135°C. The product of the digestion was allowed to slowly evaporate to near dryness. At the end of complete digestion, the digests (blood and meat) were cooled and subsequently filtered through Whatman (no. 98) filter paper into 100 mL volumetric flask and made up to mark with deionised water.
2.3. Analysis of Samples for Heavy Metals
- The concentration of heavy metal (Pb, Cd, Cr and Ni) residues in blood and meat was determined by atomic absorption spectrophotometric method [19]. The atomic absorption spectroscopy determinations of Cd, Cr, Ni and Pb, corrected for the background by means of the Zeeman effect, was carried out with an AA model buck scientific 2010, equipped with multi- element hollow cathode lamps, Analyst AA 300/Zeeman software for electronic processing of the results and a Varian PSD auto sampler. The lamp current was automatically selected for each metal. The flame conditions were optimized for maximum absorbing and linear response while aspirating known standards. For this investigation, graphite tubes of the ‘‘Varian, Partition Tubes (coated) GTA’’ type was used.
3. Results and Discussion
3.1. Results
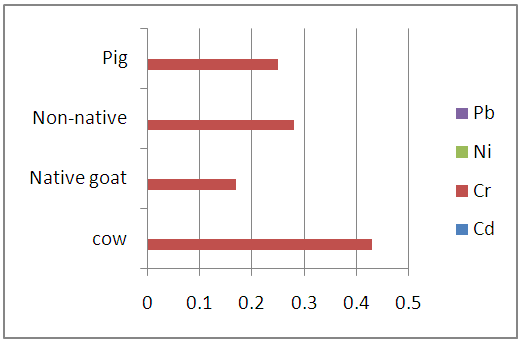 | Figure 3.1. Heavy metal levels in the blood samples of studied animals (mg/L) |
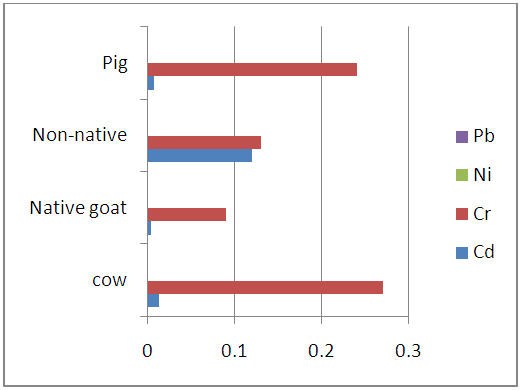 | Figure 3.2. Levels of heavy metals in the liver samples of studied animals (mg/kg) |
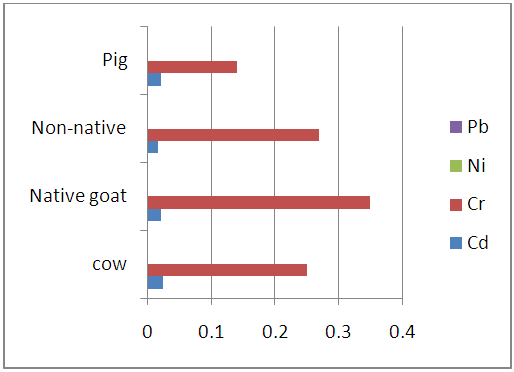 | Figure 3.3. Levels of heavy metals in the kidney samples of studied animals (mg/kg) |
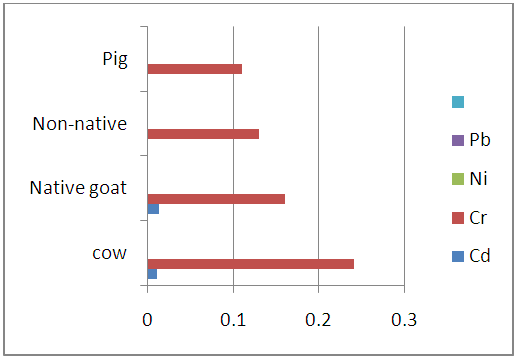 | Figure 3.4. Heavy metal levels in chitterlings samples of studied animals (mg/kg) |
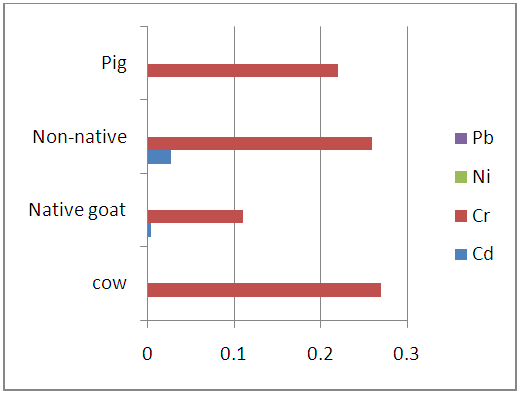 | Figure 3.5. Heavy metal levels in the stomach of studies animals (mk/kg) |
3.2. Discussion
- From the results presented in the figures above there was clear trend in the distribution of heavy metals in the entrails of the studies slaughtered animals (either within the same or from one animal to another). Among the studied heavy metals, Nickel (Ni) and Lead (Pb) were not detected in the studied samples but but Cadmium (Cd) and Chromium (Cr) were however found present within the instrument’s detection limit.
3.2.1. Intra Organ/Tissue Mean Levels of Heavy Metals in Studied Samples
- The mean concentration of Cd in cow entrails ranges from (0.023±0.018 to 0.010±0.006 mg/kg) with its highest amount occurring in the kidney while the Cr levels ranges from 0.43±0.050 mg/kg in the liver to 0.24±0.054 mg/kg in the blood of slaughtered cows. The levels of Cd were found to significant but Pb not significantly different at (p<0.05) level. In the entrails of goat (native and non-native) samples, Cd was again found to have its highest (0.020±0.006 and 0.150±0.043 mg/kg) concentration in the kidney. The least levels of cadmium were however observed in the liver, stomach and chitterlings for the slaughtered goat samples. Also the level of Cr in entrails of slaughtered goat samples ranges from 0.35±0.018 mg/kg in chitterlings to 0.11±0.006 mg/kg in the stomach samples of native goats and 0.31±0.036 mg/kg in kidney to 0.13±0.029 mg/kg in the blood samples of non-native goats. In each case, the levels of cadmium in the entrails of slaughtered goats was found to be significant while Pb levels were found to be insignificant at (p<0.05) levels.Furthermore, the study shows that Cd levels in the entrails of slaughtered pig samples was detected only in the kidney (0.020±0.012 mg/kg) and liver (0.007±0.004 mg/kg) with no significant difference at (p<0.05) level. The amount of Cr in the same samples ranges from 0.25±0.018 mg/kg in the liver to 0.11±0.027 mg/kg in the blood and were significantly different at p<0.05. In all the studied samples, the levels of cadmium detected were below the FAO/WHO permissible limit of 0.5 mg/kg for meat of livestock. However, the Cr content of all entrails samples fell short of the 0.1 mg/kg permissible limit except for native goat which had its Cr content below the FAO/WHO permissible limit [21].The high levels observed in kidney agree with several studies which have shown that cadmium bioaccumulate more in kidney than in other parts of body [18, 22, 23]. Rodriguez et al., have suggested that the higher concentration of cadmium in kidney tissue is due to the detoxification function of the organ [24] and its rate of elimination from this organ could be relatively low [25]. This is partly due to binding of Cd to sulfhydryl groups in the protein metallothione in the kidney [27]. Animals exposed to cadmium accumulate it in their kidneys because of the presence of free protein-thiol groups which leads to a strong fixation of heavy metals [25]. Pompe-Gotal inferred that the excretory mechanism for such metals, which is based on low molecular compounds with - SH groups, were poorly developed in vertebrates and could not cope with the present level of today’s anthropogenic pollution [24]. Moreover, herbivores, birds as well as mammals, demonstrate generally higher renal cadmium than carnivores since vegetation is contaminated by aerial deposition or by absorption of cadmium from the soil [28]. A trend has been reported in literature by Pearson et al., where it was shown that kidney contain high concentration of Cd [29]. His result is in agreement with previous studies in sheep and horses [30] which indicate that this tissue is one of the main organs for Cd accumulation. Cr recorded highest in the liver of cow and pig. By nature, animal liver is a natural source of essential element, such as Cu, Mg, Zn and Mn. However, liver contains higher amounts of Cr and other contaminants, which tend to accumulate in liver tissues [31]. Studies by Anderson who supplemented pigs weighing between 30 and 60 kg with 0.3 mg/kg Cr led to increased Cr levels in the liver (5.9 vs. 8.8 mg/kg), but Cr content in muscle tissue did not exceed 1.5 mg/kg disregarding the supplementation [32]. Liver records highest concentration because of its inability to filter off toxins completely as it is the organ that filters toxins from our bodies [33]. The liver is a target tissue for monitoring metal contamination in animals because the organ function in removing toxic metals from the body and therefore end up accumulating [34].High Cr content in the chitterlings of goat may be related to the physiological role of the small intestine as the active site of digestion. The small intestine is lined up with finger like projections called villi which allow the assimilation of digestive end products to the body. It appears there is a ‘trap’ in the small intestine that may accumulate heavy metals [35].
3.2.2. Variation in Mean Heavy Metal Levels in Studied Samples of Slaughtered Animals
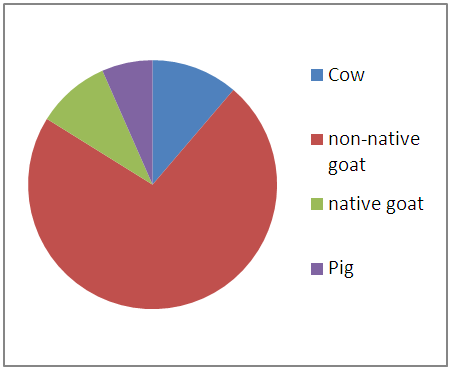 | Figure 3.6. Summation of Cd levels in entrails of animals |
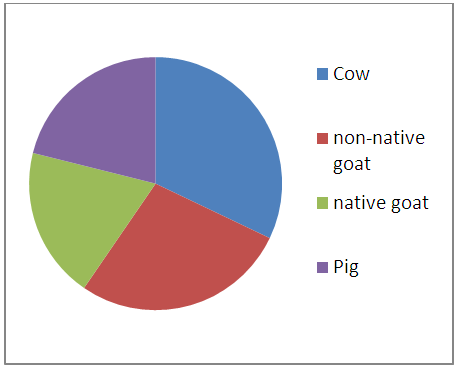 | Figure 3.7. Summation of Cr levels in entrails of animals |
4. Conclusions
- The study gave useful insight about the levels of some heavy metals in the entrails of cow, goat and pig slaughtered at Wurukum abattoir. Cadmium and Chromium were found present while the presence of Lead and Nickel was below detection limit of the instrument. The amounts of Cd in the studied samples was found to be below the FAO/WHO permissible limit while Cr concentration was found to exceed the limit set by the regulatory body. The highest amount of heavy metal concentration occurred in the kidney and liver of the slaughtered animals.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML