-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2017; 7(1): 1-6
doi:10.5923/j.aac.20170701.01

Analytical Methods Comparison for the Determination of Bilirubin in Blood Samples of Neonates
Audu I. Stephen1, Simon T. Ubwa1, Ogbene G. Igbum1, Stephen S. Hati2
1Department of Chemistry, Benue State University, Makurdi, Nigeria
2RR&P, Nisa Premier Hospital, Abuja, Nigeria
Correspondence to: Stephen S. Hati, RR&P, Nisa Premier Hospital, Abuja, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

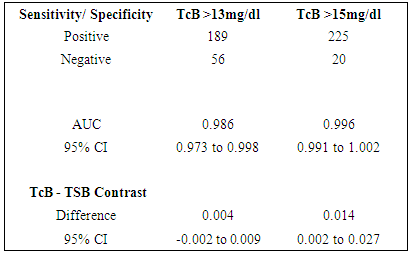
Analytical methods comparison for the determination of bilirubin in blood samples of neonates was compared to facilitate reliable clinical decision, detect hyperbilirubinemia (neonatal jaundice) or kernicterus in neonates. Details of analytical features such as accuracy, speed, sensitivity, specificity, the robustness of the methodologies/techniques compared are not well presented in some cases. In this prospective study carried out at Neonatal Special Care Unit (NSCU) of Nisa Premier Hospital Abuja, two methods: Total Serum Bilirubin (TSB) determination by direct spectrophotometry and Transcutaneous Bilirubinometry (TcB) by Jaundice Meter were compared using paired data of blood samples analysed respectively from 245 neonates<36 weeks. Data analysis was performed using Analyse-it® version 4.6 method validation software for Microsoft Excel. The overall mean value obtained by TcB (10.64±2.97mg/dl) was higher than the TSB (10.22±2.80 mg/dl), which was significantly different (ANOVA, p<.000). Bland-Altman analysis demonstrated a good agreement between TcB and TSB measurements (r = 0.976) but with an overestimation by TcB compared with evaluation with TSB (calculated by TcB-TSB), which was 2.95±17.84 mg/dl (95% CI = -0.85mg/dl to 1.70mg/dl). Relevant cut-off values were assessed and revealed that at 15mg/dl TcB provided 100% sensitivity (Area under the Curve - AUC = 99.6%) and 80% specificity. However at 13mg/dl TcB provided 98% sensitivity (AUC = 98.6%) and 66% specificity. It can be concluded that the use of TcB for bilirubin measurement in clinically jaundiced neonates is a rapid analytical methodology and the suitable alternative of TSB. However for safest clinical decisions bilirubin measurement by HPLC remains the gold standard methodology and is advocated.
Keywords: Neonates, Total Serum bilirubin, Transcutaneous bilirumetry, Neonatal jaundice
Cite this paper: Audu I. Stephen, Simon T. Ubwa, Ogbene G. Igbum, Stephen S. Hati, Analytical Methods Comparison for the Determination of Bilirubin in Blood Samples of Neonates, Advances in Analytical Chemistry, Vol. 7 No. 1, 2017, pp. 1-6. doi: 10.5923/j.aac.20170701.01.
Article Outline
1. Introduction
- Bilirubin is an endogenous, tetrapyrrolic compound, [1, 2] a reductase catalysed product of biliverdin; the cleavage of alpha-methene bridge of heme by membrane-bound heme oxygenase produces biliverdin as one of the breakdown products [3, 4]. The primary mammalian, “yellow”, bile pigment consists of unconjugated bilirubin (UCB), similar to many weakly-polar and poorly-soluble compounds; UCB is transported in blood tightly bound to albumin, with <0.01% of total serum bilirubin (TSB) circulating as free bilirubin in an unbound form [1, 5]. This is the portion of UBC that is responsible for its diffusion into tissues and consequently for its beneficial as well as toxic effects on cells. [2-4, 6]Hyperbilirubinemia (neonatal jaundice), kernicterus is a common neurotoxic condition in neonates caused by high levels of free bilirubin in the plasma. [6, 7] Although it is argued that no established safe level of bilirubin is defined, [6] more so, due to neonates’ risk for developing bilirubin neurotoxicity about the age of an infant in hours (24–96 hrs.) and the amplified bilirubin production rates in some infants, emergent preventive approaches as well as non-invasive technologies to identify and treat infants with hyperbilirubinemia have been reported. [7-10]However, to aid reliable clinical decision, and prevent kernicterus in neonates, the accuracy, speed, sensitivity, specificity, the robustness of the several analytical methodologies/techniques for measuring bilirubin concentration have been compared. Two premises of the methods are predicated on the fact that the level of unbound (or "free") bilirubin has a better sensitivity and specificity than TSB, [6] hence the development of Transcutaneous Bilirubinometry (TcB). [11]TcB measurements are non-invasive, typically by a hand-held device that allows for a quick estimate of bilirubin concentration. [12] TSB determination is regarded as the standard method of bilirubin measurement requiring blood specimen collected by venepuncture or heel prick which is time-consuming and can cause pain in the newborn. [13, 14] Hitherto clinical and non-instrumental methodology, which involves visual assessment of yellowness for the measure of bilirubin in infants, is still in use. It is thought to be the first line measure of bilirubin in neonates at risk. [15-17]Several studies have been conducted to compare these three methodologies in various combinations and for varying reasons. These include a comparison between visual assessment and TcB/TSB, [16-19] inter-TcB, [20-23] inter-TSB, [24-27] and between TcB and TSB. [13, 14, 28-52].In Nigeria, available literature suggests dearth of information concerning method comparison for bilirubin measurement in neonates as very few studies [32, 50] have been reported. In the recent study by Kayode-Adedeji et al., [32] a valid argument was raised regarding performance bias of TcB on black preterm neonates, buttressed by other studies. [14, 28] Again, in their study [32] results of TcB and TSB comparison as in some studies were presented with weak approaches lacking the statistical features of method comparison such as method reproducibility and repeatability; only correlation values formed the basis of comparison. This study aims to compare TcB and TSB methodologies in measuring bilirubin in neonates, present detailed statistical features of the methods and provide further literature base on the performance of TcB on black neonates.
2. Materials and Methods
2.1. Study Setting and Design
- The study was carried out at the Neonatal Care Baby Unit (NCBU) of Nisa Premier Hospital Abuja, Nigeria. Nisa is a renown private hospital for invitro fertilisation (IVF) and a brand for women and childcare. [53]A prospective study design was conducted between the period October 2015 and September 2016, based on a monthly average of 40 TSB determinations performed at the Bioscience Laboratory of Nisa. The study was approved by the Research Ethics Committee of the Hospital.
2.2. Subjects and Sampling
- Subjects were included in the study consecutively where clinical jaundiced preterm neonates have been identified, the gestational age<36 weeks and consents given by parents. Neonates that started phototherapy before blood sampling were excluded. Follow up was made to exclude neonates data in which Rhesus (Rh or ABO), liver, congenital malformations and haemoglobin related diseases were indicated. [14]
2.3. Determination of Bilirubin
- Transcutaneous bilirubin (TcB) was determined using TJ-20 Jaundice Detector (ANK Medical Technology, China). The TJ-20 detector is a self-calibrated device with measurement range: 0.0mg/dL~30.0mg/dL; and measurement accuracy: Low+1.0mg/dL (+17μmol/L), rest ± 1.5mg/dL (± 25.5μmol/). [54] Replicates of bilirubin measurements were taken over the infants’ forehead and sternum. [31, 32, 55, 56]Total serum bilirubin (TSB) was determined using KENZA 240TX (Biolabo, France) automated spectrophotometric biochemistry multi-tests analyser fitted with 60 independent reagent positions; up to 50 positions for samples, controls & standards; and integrated Windows® computing systems. [57]. TSB sampling was performed immediately after TcB measurement, and venepuncture or heel prick collected blood samples (up to 1ml). [13, 14]
2.4. Data Analysis
- Data collected were collated on Microsoft Excel spread sheet and analysis was performed using Analyse-it® Method Validation Edition (version 4.6, Analyse-it Leeds, UK). [58]The model rule for method comparison considered TSB as reference standard on x-axis and decision rule for the level of significance was considered at p<0.05 (95% confidence level).
3. Results
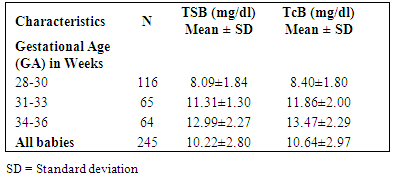
- A total of 245 neonates were sampled and 735 paired data was analysed in this study. Table 1 shows that the proportion of neonates in the GA group 28-30 weeks was the majority (116, 47%). It also indicated that higher GA of infants reflected corresponds with higher values of bilirubin concentrations in samples analysed.
|
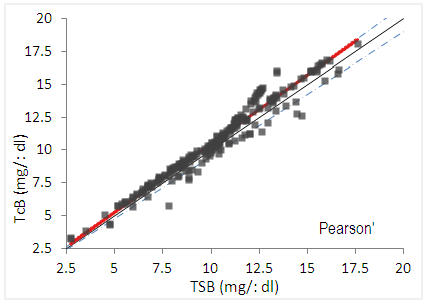 | Figure 1. Pearson’s correlation plot |
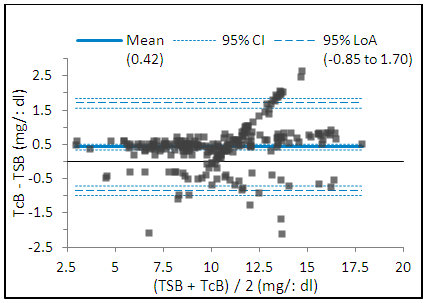 | Figure 2. Bland-Altman plot comparing TcB-TSB measurement |
|
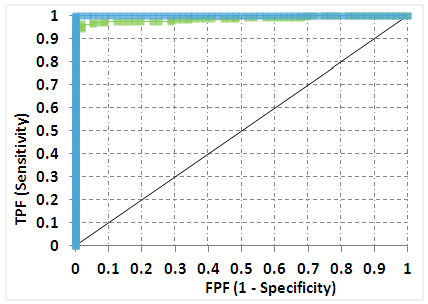 | Figure 3. Receiver operating characteristic (ROC) curve for TcB measurements at > 13mg/dl |
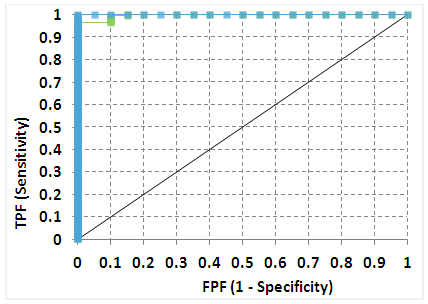 | Figure 4. ROC curve for TcB measurements at > 15mg/dl |
4. Discussion
- The review of literature enumerated in this study shows that comparison between TcB and TSB methodologies is huge and will remain an unending endeavour, at least for monitoring purposes and to buttress already established positions. Notwithstanding the innovations to make TcB devices a more reliable alternative, such as the technological improvements that interfaces the device with electronic medical records, [59] differences still exit. Findings of this study which generally indicated a higher bilirubin measurements by TcB than TSB, correspond with findings of many other studies. [14, 30–32] It was inferred that this might be because TcB measures bilirubin in extravascular tissue other than that measured in the blood by TSB, [10, 60] leading to improved sensitivity and specificity. [6]The significantly different mean values between the TcB and TSB results by ANOVA, and the high level of agreement by Bland-Altman analyses have also been observed in similar studies. [10, 14, 30, 32] Although the statistical tests explained different features, but a significant objective of an analytical method, especially in clinical practice, is to preclude or drastically minimise the error of missing a reading in which high bilirubin concentrations should have been detected and this would necessitate the appropriate therapeutic intervention, which is expensive and time-consuming, such as phototherapy or even exchange transfusion to reduce plasma bilirubin concentrations. [25, 36] According to Puppalwar et al., [61] jaundice can usually be detected when the serum bilirubin level exceeds 2.0 to 2.5 mg/dl. Although it has been reported that between 25 and 50% of all term newborns develop clinical jaundice, and a serum bilirubin level >15 mg/dl is found in 3% of normal term infants. [62]Some factors have been considered to influence TcB measurements. This include the gestational age, [63] appropriate measurement site for TcB; recommended over sternum or abdomen in premature infants born at 32-34 gestational weeks [55] from the forehead to the sternum with advancing postnatal age. [56] Disease conditions such as congenital malformations liver and haemoglobin related diseases and Rhesus (Rh or ABO) are also very likely to affect both readings of TcB or TSB. [14]Inter instrumental variability of TcB has been made indicating non-significant interinstrumental variability. [20] However, in this study, similar to the reproducibility of Palmer [43] the replicate data for TcB provided coefficient of variation (CV) of 4.5% with a 75.8% chance of the machine giving the exact value second generation bilirubin meter, such as Draeger JM-103® device. [35]Although the correlation between the two methods was significant (r = 0.66, P < 0.01), the scatter was wide, and the specificity of only 53% in other studies [40, 44] are within the same performance level as determined in this study.That a good correlation was found between the TcB and TSB in this study correlation was 0.774. The observed sensitivity was 90%, specificity 78% and positive predictive value 64% at mean serum bilirubin concentration of 15mg/dl. Grohmann et al., [31] suggested that in the routine care of newborns, the first method for bilirubin testing should be a skin test. If the skin test result exceeds 200µmol/L and other analytes are to be determined with a nonchemical photometric device, then bilirubin can be included in this analysis.It should be noted that while high-performance liquid chromatography (HPLC), which is the gold standard for bilirubin measurement, [14] spectrophotometric methods have been widely used and regarded as gold standard.On the whole, while this study posits the use of TcB as suitable alternative to TSB, similar the position of other studies, [14, 30-32] the TcB meter compared with systematic visual assessment of bilirubin significantly reduced the need for blood sampling to assay STB in jaundiced neonates. [17, 64] but Neocleous et al., [14] observed contrary to this position that TcB values did not correlate well with TSB values, being often imprecise in predicting the actual TSB levels.
5. Conclusions
- In the present study, regarding method comparison between TcB and TSB bilirubin measurement values which correlated well and although TcB values negligibly provided imprecision in predicting actual TSB levels. This finding permits us to support the safe use of TcB to decide on a care plan for clinically jaundiced neonates.This study also agrees with the concluding position that TcB measurement devices could be used in rare instances if the limits of agreement between TSB and TcB levels are considered, and clinical judgement is sustained.
ACKNOWLEDGEMENTS
- Authors acknowledge the support of the Neonatology Department and Bioscience Laboratory of Nisa Premier Hospital, Abuja Nigeria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML