-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2016; 6(2): 41-48
doi:10.5923/j.aac.20160602.02

Development of an Electrochemical Assay for the Illegal “Fat Burner” 2,4-dinitrophenol and Its Potential Application in Forensic and Biomedical Analysis
Kevin C. Honeychurch
Department of Applied Sciences, Faculty of Health and Life Sciences, University of the West of England, Frenchay Campus, UK
Correspondence to: Kevin C. Honeychurch, Department of Applied Sciences, Faculty of Health and Life Sciences, University of the West of England, Frenchay Campus, UK.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The illegal use of 2,4-dinitrophenol (24DNP) as a diet aid has increased markedly resulting in a number of deaths. This paper describes the development of a simple voltammetric method for the measurement of 24DNP in serum at glassy carbon electrode (GCE). It is believed that this is the first report on the voltammetric determination of 24DNP in any biological sample. Initial investigations were undertaken using cyclic voltammetry to characterise the redox behaviour of 24DNP. Over the pH range 2 to 10 four pH dependent reduction peaks were recorded on the initial negative going scan, concluded to result from the reduction of the two nitro groups to the corresponding hydroxylamines. On the return positive going scan two oxidation peaks were recorded, resulting from the oxidation of the hydroxylamine (O1) formed on the initial negative scan and the direct oxidation of the phenol group (O2). At pH 6, the peak potential of the oxidation process O1 occurred at a potential close to 0 V and was chosen for investigation. The optimum voltammetric conditions required were identified to be supporting electrolyte of 0.1 M pH 6.0 phosphate buffer containing 10% acetonitrile. Using differential pulse voltammetry, the calibration plot was found to be linear from 180 ng/mL to 184 µg/mL (R2= 0.9996), with a detection limit of 98.4 ng/mL (based on a signal-to-noise ratio of 3). The optimised method was evaluated by carrying out 24DNP determinations on spiked and unspiked serum samples. Using an external calibration technique, mean recoveries of 79.2% were obtained and coefficients of variation of 7.4% were calculated for a forensically relative concentration of 36.8 µg/mL. The performance characteristics show that the method holds promise and reliable data may be obtained for 24DNP in forensics and bioanalysis.
Keywords: Voltammetry, Serum 2,4-dinitrophenol
Cite this paper: Kevin C. Honeychurch, Development of an Electrochemical Assay for the Illegal “Fat Burner” 2,4-dinitrophenol and Its Potential Application in Forensic and Biomedical Analysis, Advances in Analytical Chemistry, Vol. 6 No. 2, 2016, pp. 41-48. doi: 10.5923/j.aac.20160602.02.
Article Outline
1. Introduction
- 2,4-dinitrophenol (24DNP) has been utilised for a number of different applications including the manufacture of dyes, pesticides, explosives and as a dieting aid. Frist World War French munition workers working with 24DNP reported symptoms of weight loss, weakness, dizziness, excess sweating associated with its exposure, resulting in some cases with death [1]. Further investigations showed that low doses of 24DNP increased metabolic rate resulting in weight loss; observations which lead in the 1930s to 24DNP being marketed as a diet-aid [2, 3]. However, once freely available unsupervised usage resulted in a number of deaths and symptoms similar to that reported by the French munition workers. Furthermore, young women taking 24DNP under medical supervision starting losing their eyesight; a situation latter seen in the wider United States populace [2]. Due to the wide range of adverse symptoms and deaths reported the American Food and Drug Administration banned its use for humans in 1938. Nevertheless, in spite of this, reports of its usage continued with 24DNP recently being again marketed as a dieting aid, principally via the internet [4]; resulting in the occurrence of the same range of adverse side effects and a number of deaths [4-13] with blood 24DNP levels in fatalities ranging from 28 mg/L [6] to 144 mg/L [14]. Accordingly, there is a pressing need for methods that can detect 24DNP in complex matrixes such serum. Principally methods have focused on liquid chromatography mass spectroscopy [6, 15] or gas chromatography mass spectroscopy [14]; techniques which are expensive, requiring highly trained staff and well-equipped laboratories for their implementation. Electrochemical techniques such as voltammetry have been shown to be inexpensive, sensitive and can be used on-site, at the point of need. Most modern electrochemical instrumentation can be run on internal battery power and controlled via laptop computer. Previously, the voltammetric behaviour of a number of nitro aromatic compounds has been investigated [16-20], including 4-nitrophenol [21] and 2,6-dintrotoluene [22] in serum and saliva using such an approach. Consequently, it was considered feasible to develop an electrochemical method for the determination of 24DNP in a biological fluid such as serum. Table 1 describes previous electrochemical based methods for the determination of 24DNP. However, to our knowledge this is the first report on the exploitation of the voltammetric redox behaviour of 24DNP for its determination and is the first example of its electrochemical determination in any biological sample. In this study the cyclic voltammetric behaviour of 24DNP is first discussed. In the second section the optimisation of the conditions necessary for the differential pulse voltammetric (DPV) determination of 24DNP in serum are investigated and optimised.
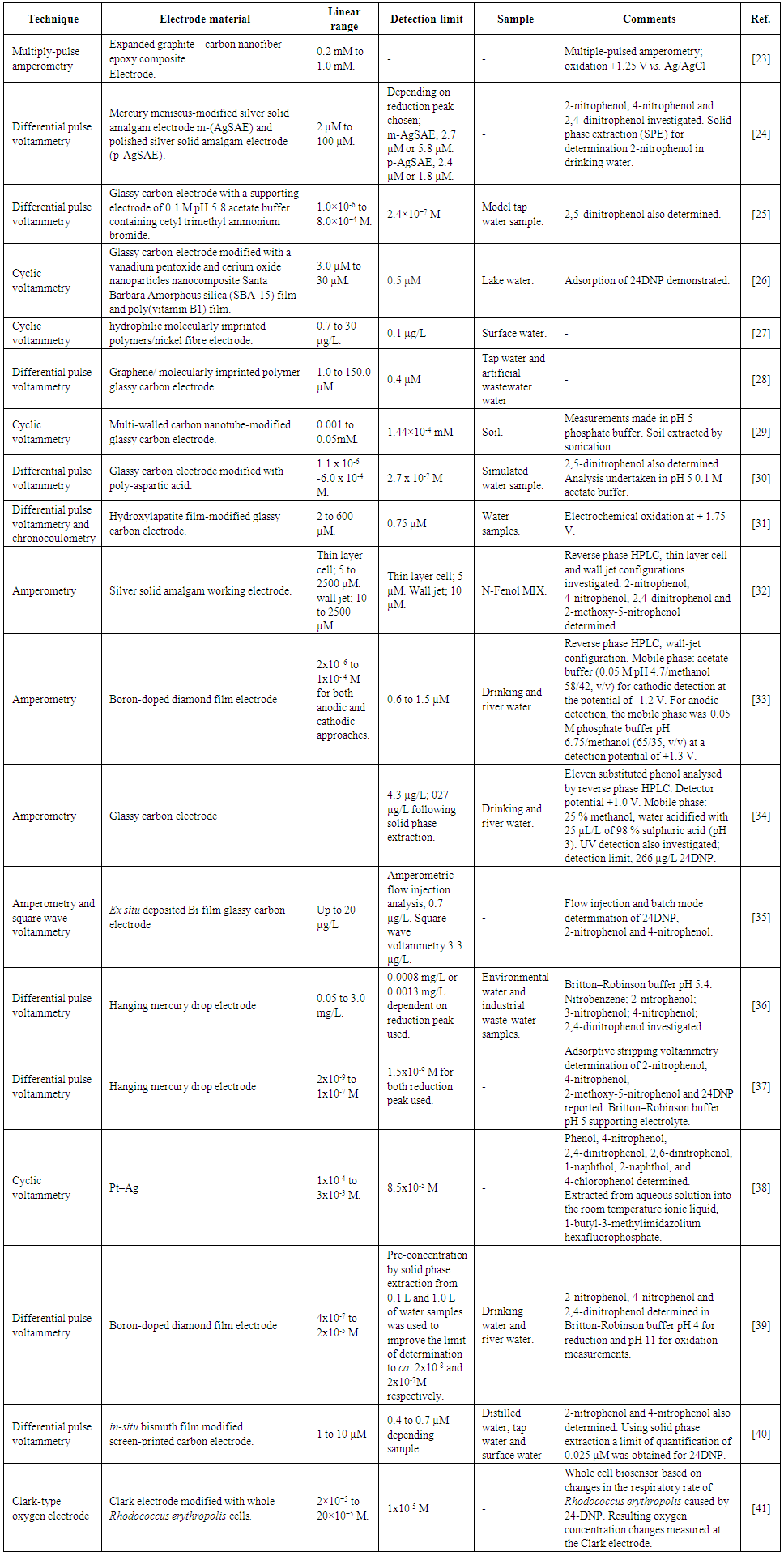 | Table 1. Electrochemical based methods for the determination of 2,4-dinitrophenol |
2. Experimental
2.1. Chemicals and Reagents
- All chemicals were obtained from Fischer (Loughborough, UK), unless otherwise stated. Deionised water was obtained from a Purite RO200-Stillplus HP System, (Purite Oxon., UK). Stock solutions of sodium orthophosphate were made at a concentration of 0.2 M by dissolving the appropriate mass of solid in deionised water to give a 0.2 M solution. A stock solution of orthophosphoric acid was made by dilution in deionised water. These were then titrated together to give the required pH. Stock solutions of 2,4-dinitrophenol were prepared by dissolving the required mass in acetonitrile to give a concentration of 10 mM. Working standards, for voltammetric studies, were prepared by dilution of the primary stock solution with phosphate buffer and acetonitrile to give a final concentration of 0.1 M phosphate buffer. These were then adjusted with sufficient water to give a 10% acetonitrile.
2.2. Apparatus
- Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were undertaken using an Ivium CompactStat potentiostat (Ivium Technologies, The Netherlands) interfaced to a PC for instrument control and data acquisition. The voltammetric cell contained a platinum wire auxiliary electrode, a Ag/AgCl reference electrode, and a glassy carbon electrode (GCE) (3 mm diameter), as the working electrode. The GCE was cleaned before each scan by polishing with slurry of aluminium powder. Cyclic voltammograms were initially recorded with plain solutions of 0.1 M phosphate buffer, containing 10 % acetonitrile and then in the same solution containing 1 mM 24DNP. Samples were purged with nitrogen (BOC, Guildford, UK) for 10 minutes to eliminate oxygen. A starting and end potential of 0.0 V, an initial switching potential 1.5 V, second switching potential +1.0 V were utilised. Differential pulse voltammetry was undertaken using a starting potential of 1.3 V and a final potential of 0.0 V; using a step height of 10 mV, pulse repetition time 0.2 s, pulse amplitude of 75 mV, and pulse duration of 50 ms.
2.3. Analytical Application
- A 1.0 mL aliquot of bovine serum was taken and diluted ten times to be 10% acetonitrile in 0.1 M phosphate buffer pH 6.0. A second aliquot was fortified to give 36.8 µg/mL 24DNP, and then diluted ten times to give a 10% acetonitrile and 0.1 M phosphate buffer pH 6.0 solution. These were then examined using the optimised differential pulse voltammetric procedure.
3. Results and Discussion
3.1. Voltammetric Studies of 2,4-Dinitrophenol at Glassy Carbon Electrode
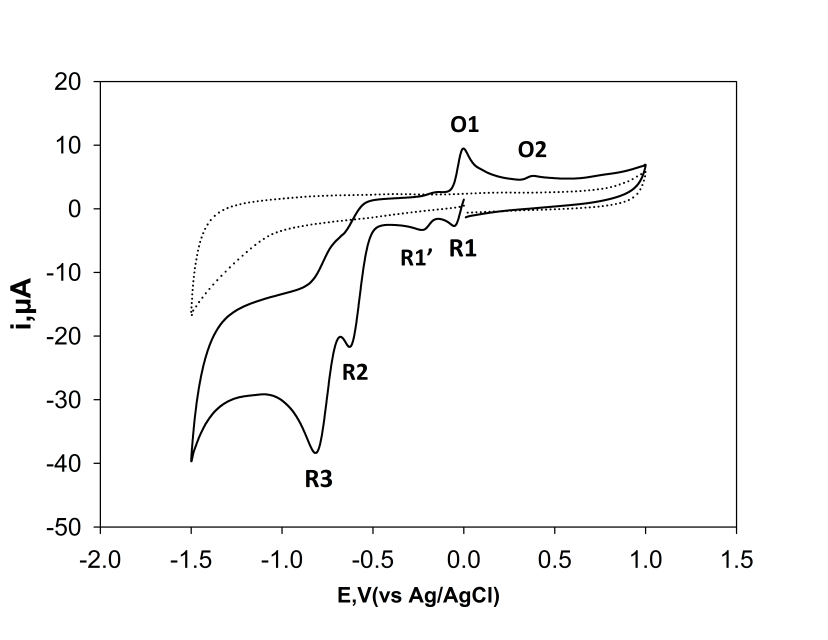
- Initial cyclic voltammetric studies were made with a 1 mM solution of 24DNP, dissolved in 0.1 M phosphate buffer pH 6, containing 10% acetonitrile (10 mL). Figure 1 shows the resulting voltammogram obtained using a scan rate of 50 mV/s. This shows the appearance of four reduction peaks, R1, R1’, R2 and R3 on the first forward scan. The Ep of the first two reduction peaks R1 and R1’ were both found to be independent of pH; indicating that no protons were involved the electrochemical process. Consequently, these peaks were considered to result from the one electron reduction of the two nitro groups to form a nitro radical anion (eq. 1) [42]. The two further reduction peaks R2 and R3 result from the formation of hydroxylamine moieties (eq. 2) formed form the sequential reduction of the two nitro groups. This is in agreement with polarographic studies made by Taha et al [43] who concluded that the two nitro groups of 24DNP are reduced independently of each other. On the return scan, two oxidation peaks O1 and O2 are observed. The oxidation O1 was considered to be the result of oxidation of the hydroxylamine species formed on the initial negative going scan to give the corresponding nitrosamine (eq. 3). The second oxidation peak, O2 was concluded to result from the oxidation of the phenol (eq. 4).Further cyclic voltammetric investigations were made to explore this proposed mechanism for the formation of O1 and O2. Without prior reduction the oxidation peak O1 was not observable, however, peak O2 was still recorded. Consequently, it was concluded that O1 was the result of an oxidation of a compound formed on the negative going scan and O2 was the result of the direct oxidation of the molecule.
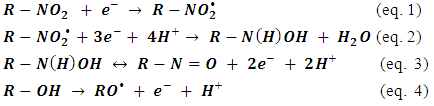
3.2. Effect of pH
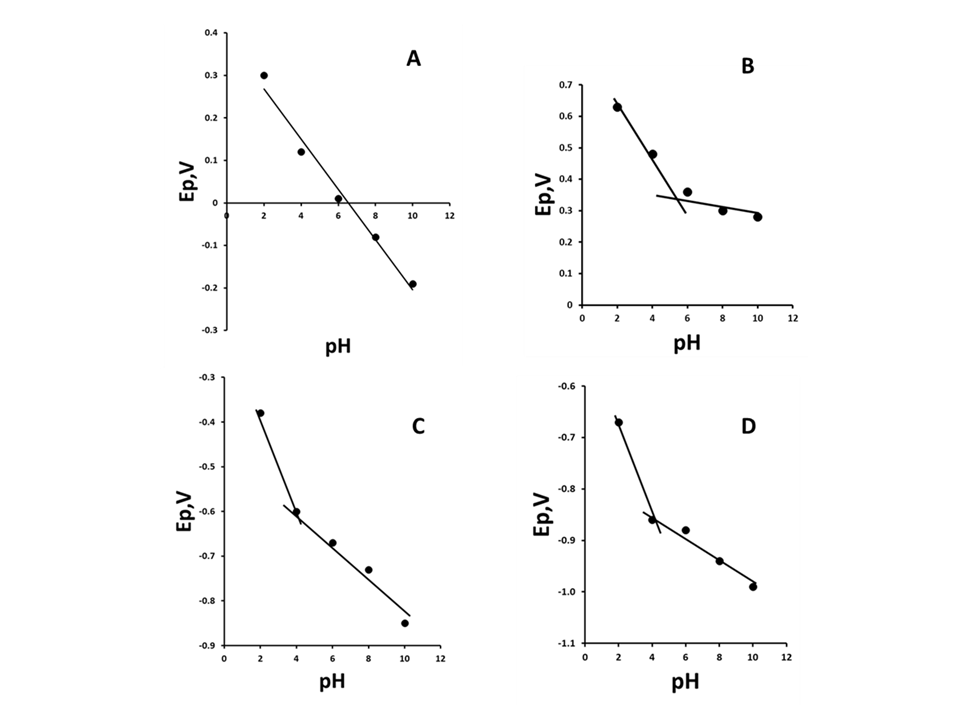
- Figure 2 shows the plots of Ep versus pH for peaks R2, R3, O1 and O2 obtained over the range pH 2–10. The oxidation peak O1 (Figure 2A) was found to be pH dependant over the entire pH range studied. A plot of Ep vs. pH was found to give a slope of 59 mV/pH, indicative of an equal number of protons and electrons involved in the oxidation step. The Ep of the oxidation process O2 (Figure 2B) was found to be dependent between pH 2 and 6. Above pH 6 the Ep of this peak was found to be constant. This was considered to be the result of an apparent pKa associated with the phenol group. Figure 2C and Figure 2D show that the reduction peaks R2 and R3 are pH dependent over the whole range investigated with slopes 60 mV/pH corresponding to an equal number of electrons and protons involved in their reduction.The peak current (ip) of all three peaks were found to be pH dependent over the range studied (Figure 3). The peak current of R2 and R3 was found to decrease with increasing pH. The peak current for O2 was found to decrease rapidly with pH and was only recordable at pH 2. This was most properly due to the stabilisation of the anion by the nitro groups. The maximum peak current of the oxidation peak O1 was found at pH 6. As this oxidation occurred at a potential close to 0 V it was chosen to investigate this peak further as this others a number of analytical advantages; such as lower background currents and is free from the common interference such as oxygen. Consequently, subsequent studies were undertaken using a supporting electrolyte of 0.1 M pH 6.0 phosphate buffer.
 | Figure 2. Plot of Ep and ip vs. pH for 2,4-dinitrophenol. Ep vs. pH A) Peak O1, B) peak O2, C) peak R1, D) R2 |
 | Figure 3. ip vs. pH, peak O1, peak R2, peak R3. Voltammetric conditions as Figure 1. Error bars represent ±σ |
3.3. Effect of Scan Rate
- The effect of scan rate was studied on the cyclic voltammetric behaviour of 24DNP at pH 6 was investigated over the range of 20 to 200 mV/s. A plot of peak height vs. the square root of scan (v½) was found to linear for all the peaks studied indicating a diffusional process. Peak potentials (Ep) were found to become increasingly more negative with scan rate suggesting electrochemical irreversibility.
3.4. Effect of Starting Potential
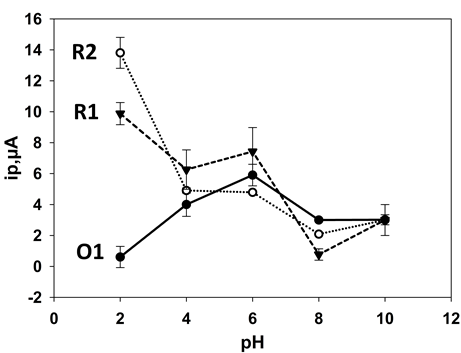
- As it was planned to develop a voltammetric method for the determination of 24DNP based on the oxidation peak O1 further investigations were undertaken by linear sweep voltammetry. Figure 4 shows the effect of starting potential on the result ip magnitude of O1 using a pre-treatment time of 20 s at pH 6. A near linear relationship with applied potential was gained between 0.5 and 1.1 V (vs. Ag/AgCl) which then plateaus at potentials more negative than this. Consequently, further studies were made using an applied potential of 1.3 V (vs. Ag/AgCl).
 | Figure 4. Effect of starting potential on 0.1 mM 2,4-dinitrophenol O1 oxidation peak obtained by linear sweep voltammetry at pH 6.0. Error bars represent ±σ |
3.5. Calibration Curve and Limit of Detection
- In this part of our investigation it was decided to use the differential pulse waveform as it offers advantages over the linear sweep waveform. Such advantages include better discrimination between faradaic current and capacitance current giving improved sensitivity and resolution. Consequently, it should be beneficial for the determination of 24DNP in real samples. Initial studies were undertaken to study the effect of 24DNP concentrations on the magnitude of the differential pulse peak O1 occurring at a potential of 0 V. The calibration plot was found to be linear from 180 ng/mL to 184 µg/mL (R2 = 0.9996), with a detection limit of 98.4 ng/mL (based on a signal-to-noise ratio of 3).
3.6. Analytical Application
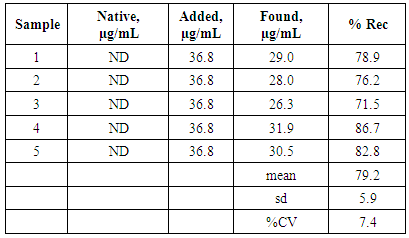
- The optimised procedure was used to determine the concentration of 24DNP in spiked and unspiked bovine serum samples. Figure 5 shows the typical voltammograms obtained for theses samples and the recovery and precision data is summarised in Table 2. Clearly, these results show that the optimised differential pulse voltammetric method offers the possibility of determining 24DNP at forensically and bio-medically [6] relevant level in real samples.
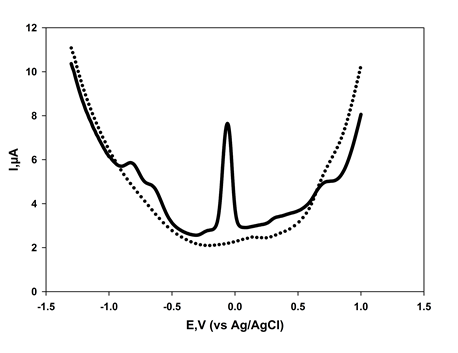 | Figure 5. Differential pulse voltammograms obtained for serum sample. Dashed line in the absence and solid line in the presence of 36.8 µg/mL 2,4-dinitrophenol |
|
4. Conclusions
- This is believed to be the first report on the utilisation of the redox generated hydroxylamine for the determination of 24DNP. It is also the first example of the voltammetric determination of 24DNP in a biological fluid. The paper demonstrates that 24DNP yields a well-defined voltammetric signal at a GCE. A simple and convenient assay for 24DNP was developed requiring only the dilution of sample in the optimised supporting electrolyte. A detection limit of 98.4 ng/mL with a linear response from 180 ng/mL up to 184 µg/mL (R2 = 0.9996). The results shown in Table 2 indicate that reliable results may be gained using this approach using an external calibration method. The proposed method was shown capable of determining 24DNP in a serum samples at concentrations reported in real forensic and biomedical investigations [6]. This approach avoids the problems associated with the high positive or negative potentials normally used for the determination of 24DNP.
ACKNOWLEDGEMENTS
- The author would like to thank The University of the West of England for funding. Both Paul Bowdler and Kevin Sudlow are thanked for their technical assistance. Jonathan Parker is thanked for undertaking some preliminary studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML